


Phase 2 trials are essential for evaluating the efficacy and safety of new therapies, representing a crucial phase in drug development that determines whether a treatment should advance to larger trials. This article delineates key strategies for success, encompassing:
These elements collectively enhance the trial's rigor and significantly elevate the probability of favorable outcomes.
Phase 2 trials mark a pivotal point in the clinical development process, where the potential of new therapies undergoes rigorous evaluation for safety and efficacy. These trials are crucial not only for determining the viability of treatments for further investigation but also for shaping the strategic direction of drug development, making their success essential.
As the complexity of these studies escalates, the challenges surrounding recruitment, regulatory compliance, and methodological design also intensify.
How can researchers effectively navigate this intricate landscape to ensure that their Phase 2 trials yield meaningful results and facilitate future medical advancements?
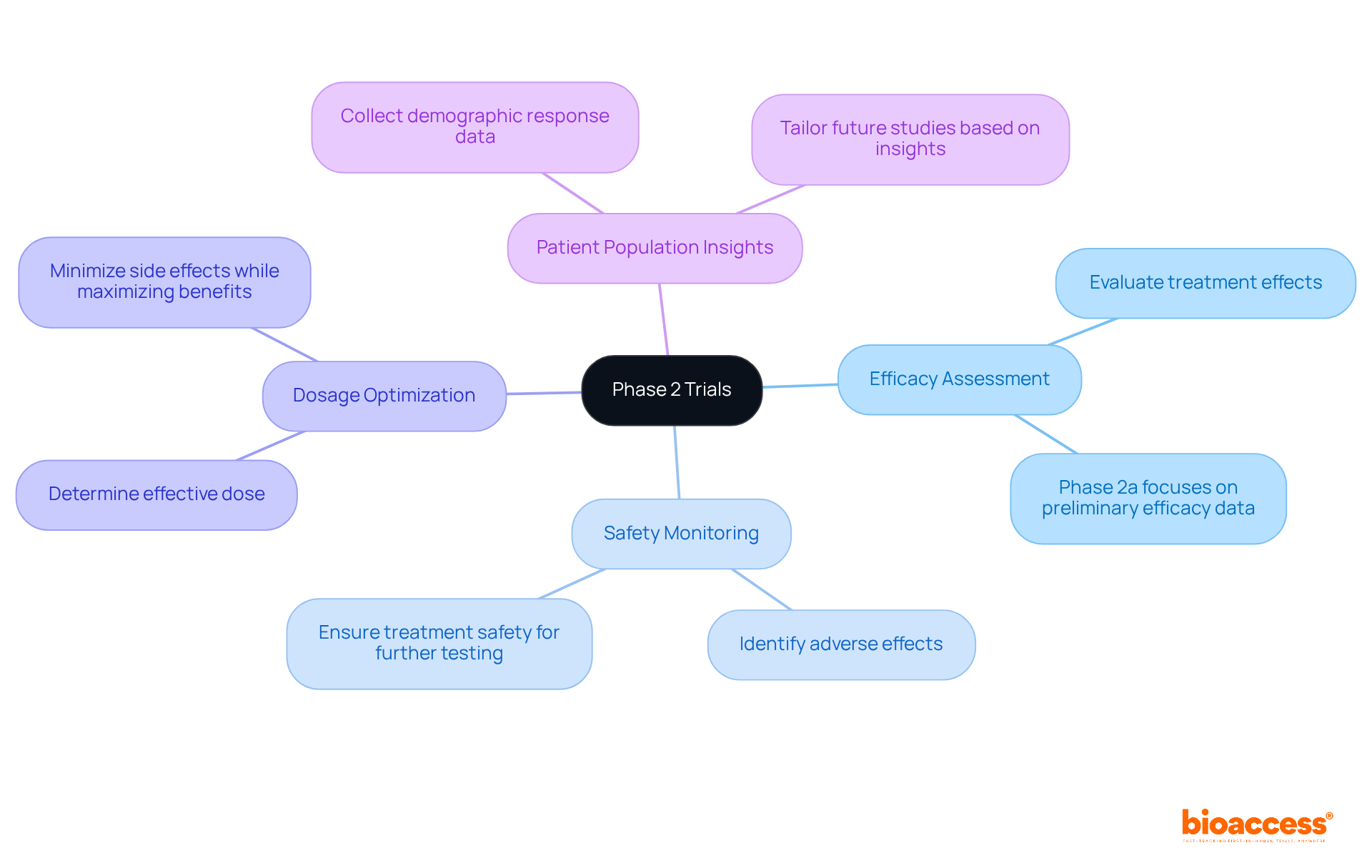
Phase 2 trial evaluations are essential in assessing the effectiveness and safety of new therapies following the preliminary safety evaluations conducted in Stage 1. Typically involving 100 to 300 participants, these studies serve several key purposes that are vital for determining whether a treatment merits further investigation in larger phase 2 trials. The outcomes of the phase 2 trial can significantly influence a drug's trajectory, marking a pivotal moment in the clinical development process.
Understanding the objectives of the phase 2 trial evaluations is critical for stakeholders, as it informs resource allocation and research frameworks, ultimately impacting the success of the drug development pipeline. Collaborating with a reputable Contract Research Organization (CRO) like bioaccess can enhance the management of these studies, ensuring compliance with regulatory requirements and optimizing execution through services such as feasibility assessments, site selection, and compliance reviews.
Key Objectives of Phase 2 Trials:
Recent advancements in the second stage of effectiveness evaluation highlight the increasing complexity of study designs, with a notable rise in the average number of eligibility requirements from 31 to 50—a 61% increase. This complexity underscores the importance of collaboration with CROs such as bioaccess to navigate compliance demands and enhance study rigor. Successful phase 2 trial studies can yield critical information that guides oversight organizations and potentially accelerates the evaluation process for promising treatments.
Case studies illustrate the objectives of Stage 2 experiments in drug development. For instance, the successful execution of the phase 2 trial studies is vital for medication approval, as regulatory bodies require robust evidence of effectiveness and safety. Furthermore, the rising costs associated with drug development, averaging approximately $2.6 billion as of 2023, emphasize the necessity for efficient second-stage evaluations to ensure that investments yield viable therapeutic alternatives. In summary, phase 2 trial studies are not merely a step in the process; they are a fundamental component of successful medication development.
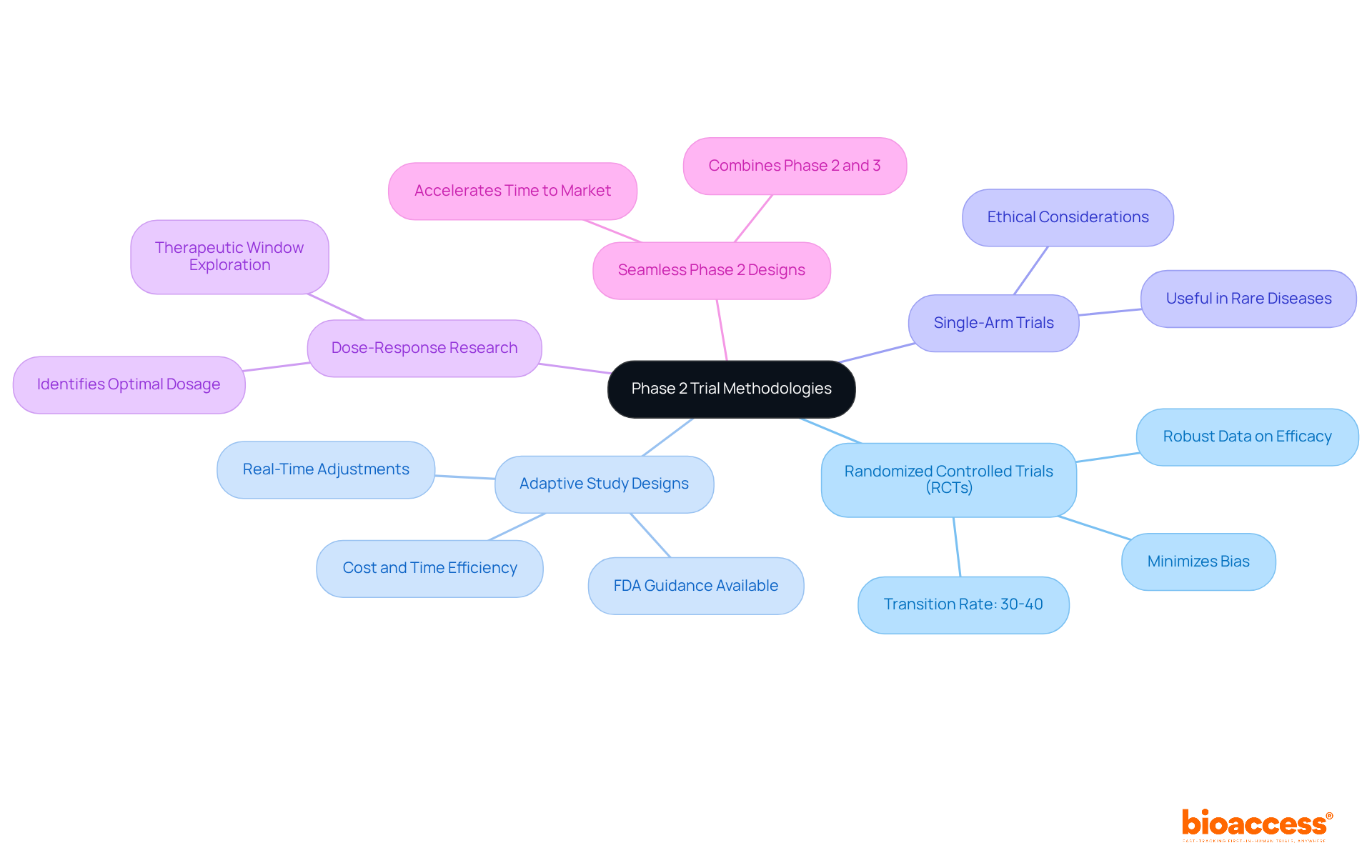
Creating Stage 2 studies necessitates a meticulous choice of methods that align with the study's goals. The following methodologies are prevalent in this phase:
Alongside these methodologies, utilizing bioaccess's expedited regulatory approval process—gaining approvals in merely 6-8 weeks—can greatly improve the practicality of study designs. Furthermore, enrolling treatment-naive cardiology or neurology cohorts 50% faster than Western sites addresses common recruitment challenges, ensuring a diverse and representative participant pool. Each methodology offers distinct benefits and difficulties, and the choice should be influenced by the study's specific goals, the nature of the treatment, and the attributes of the patient population involved. Incorporating strategies for patient engagement, as demonstrated in the case study on "Engagement with Parkinson’s Patients and Caregivers," can further enhance recruitment and retention.
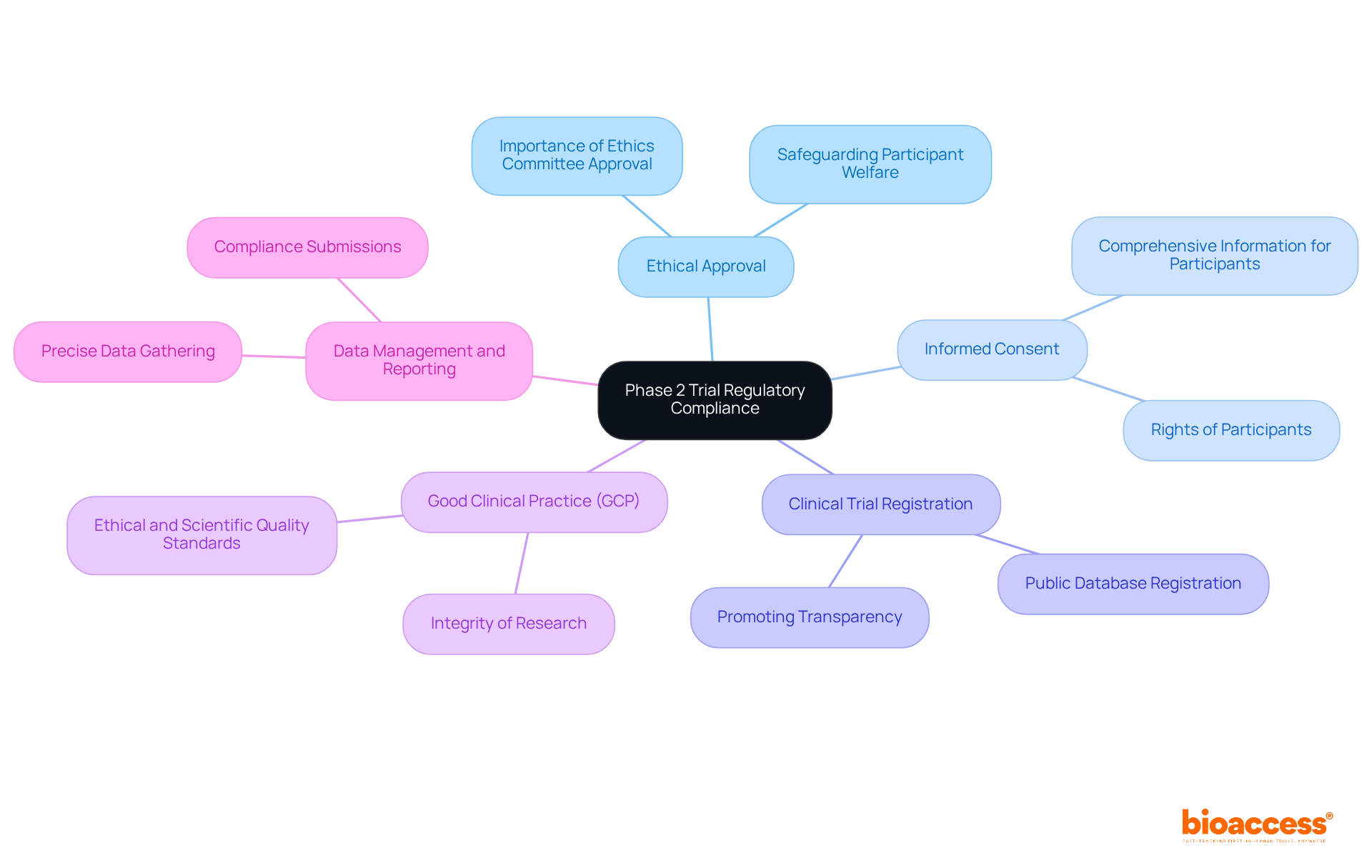
Navigating the compliance environment is crucial for the successful implementation of the phase 2 trial. Key regulatory requirements include:
In Colombia, the procedure to obtain clinical study approval involves several critical steps: securing ethical approval from the IRB, obtaining study approval from INVIMA (the regulatory agency), and acquiring an import permit from the Ministry of Industry and Commerce (MinCIT) for investigational devices. These steps are essential for ensuring adherence to local regulations and facilitating the smooth execution of tests.
In 2025, the percentage of phase 2 trial studies obtaining ethical approval remains a critical metric, reflecting the commitment to ethical standards in clinical research. Practical instances demonstrate that studies following these ethical approval procedures not only overcome compliance obstacles more efficiently but also enhance the overall achievement of medication development. Comprehending and following these compliance requirements enhances the credibility of the research findings, ultimately facilitating the drug approval process. Moreover, with the introduction of the Clinical Trials Regulation (CTR) in the EU, all clinical studies ongoing after January 30, 2025, must adhere to these updated regulations, making it essential for researchers to remain informed and compliant. Bioaccess provides extensive clinical study management services that aid in navigating these regulatory requirements, ensuring a streamlined process for successful execution.
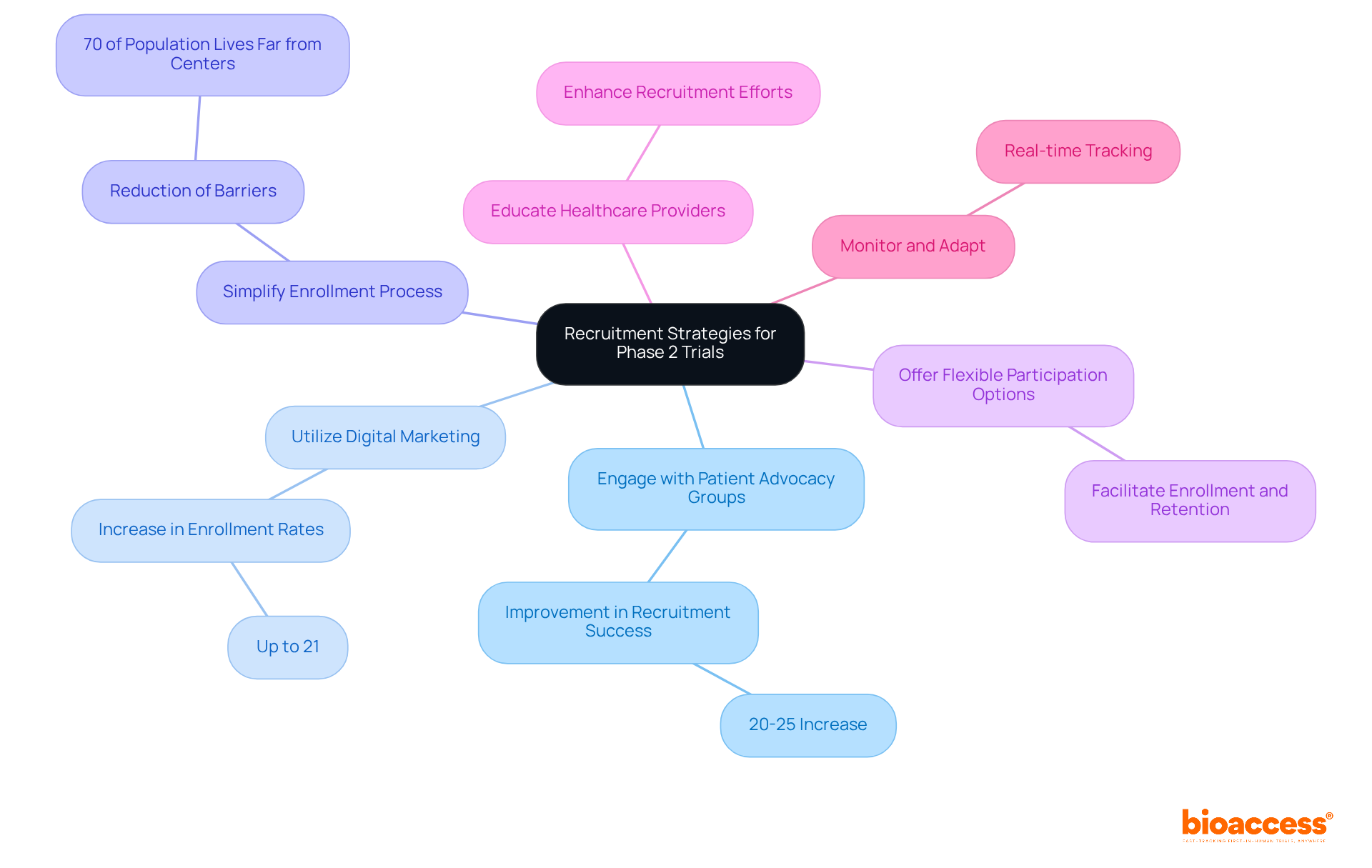
Recruitment challenges can significantly affect the timeline and success of the phase 2 trial. To enhance recruitment efforts, consider the following effective strategies:
Engage with Patient Advocacy Groups: Collaborating with organizations that represent patient populations can raise awareness about the study and encourage participation. Engaging these groups has been shown to improve recruitment success rates by 20-25%, as evidenced by recent case studies.
Utilize Digital Marketing: Leverage social media, online advertisements, and targeted email campaigns to reach potential participants. The use of digital recruitment methods has increased enrollment rates by up to 21%, making them essential in today's clinical landscape.
Simplify the Enrollment Process: Streamline the enrollment process by minimizing paperwork and providing clear instructions to potential participants. A simplified approach can significantly reduce barriers to entry, especially considering that nearly 70% of the population lives two hours or more from an academic medical center.
Offer Flexible Participation Options: Providing options for remote participation or flexible scheduling can facilitate enrollment and retention. This adaptability is crucial, particularly as many potential participants may face geographical barriers.
Educate Healthcare Providers: Informing local healthcare providers about the study can help them identify eligible patients and encourage referrals. This grassroots approach can enhance recruitment efforts significantly. Significantly, bioaccess has been proactively involved in educating providers in Barranquilla, Colombia, as part of its partnership with Caribbean Health Group to establish the city as a premier location for clinical research in Latin America.
Monitor and Adapt: Continuously track recruitment progress and be willing to adjust strategies based on effectiveness. Utilizing a Subject Recruitment and Retention Plan (SRRP) allows for real-time tracking and swift adjustments, helping to identify challenges early and allowing for timely interventions. This approach is particularly relevant given the financial constraints many medical device startups face, as highlighted in recent case studies.
By implementing these strategies, researchers can improve their chances of meeting recruitment goals, thereby enhancing the overall success of phase 2 trial.
Mastering Phase 2 trials is a critical step in the journey of drug development, serving as a bridge between initial safety assessments and larger-scale evaluations. These trials are essential for determining the efficacy, safety, and optimal dosages of new therapies, ultimately shaping the future of clinical research and patient care.
Throughout this article, key strategies for successful Phase 2 trials have been highlighted. Effective methodologies, such as randomized controlled trials and adaptive designs, are crucial, alongside the necessity of regulatory compliance and ethical considerations. Furthermore, addressing recruitment challenges through innovative strategies can significantly enhance participant enrollment and retention, ultimately influencing the trial's success.
In light of these insights, it is imperative for stakeholders in the clinical research field to prioritize the meticulous execution of Phase 2 trials. By embracing best practices, leveraging advanced methodologies, and fostering collaboration with patient advocacy groups and regulatory bodies, the potential for successful outcomes in drug development can be greatly expanded. The commitment to mastering Phase 2 trials not only benefits the research community but also holds the promise of delivering innovative therapies that can improve patient lives.
What are Phase 2 trials?
Phase 2 trials are evaluations that assess the effectiveness and safety of new therapies after preliminary safety evaluations in Stage 1. They typically involve 100 to 300 participants.
What are the key objectives of Phase 2 trials?
The key objectives of Phase 2 trials include efficacy assessment, safety monitoring, dosage optimization, and gathering insights on patient population responses to the treatment.
How does efficacy assessment work in Phase 2 trials?
Efficacy assessment in Phase 2 trials evaluates whether the treatment achieves its intended effects, with Phase 2a trials focusing specifically on preliminary efficacy data.
What is the importance of safety monitoring in Phase 2 trials?
Safety monitoring identifies any adverse effects or complications that may arise during the trial, ensuring that the treatment remains safe for further testing.
Why is dosage optimization crucial in Phase 2 trials?
Dosage optimization determines the most effective dose that minimizes side effects while maximizing therapeutic benefits, which is essential for the success of the treatment.
How do Phase 2 trials provide insights into patient populations?
Phase 2 trials collect data on how various demographics respond to the treatment, which helps tailor future studies and ensures broad applicability of the therapy.
What recent trends have been observed in Phase 2 trial designs?
There has been an increase in the average number of eligibility requirements for Phase 2 trials, rising from 31 to 50, indicating a growing complexity in study designs.
How can collaboration with a Contract Research Organization (CRO) benefit Phase 2 trials?
Collaborating with a reputable CRO, like bioaccess, can enhance the management of Phase 2 trials by ensuring compliance with regulatory requirements and optimizing study execution.
Why are successful Phase 2 trials critical for drug approval?
Successful Phase 2 trials provide robust evidence of a treatment's effectiveness and safety, which is required by regulatory bodies for medication approval.
What is the average cost associated with drug development as of 2023?
The average cost associated with drug development is approximately $2.6 billion, highlighting the necessity for efficient Phase 2 evaluations to ensure viable therapeutic alternatives.