


The FDA Diversity Action Plan (DAP) is designed to enhance the representation of historically underrepresented groups in clinical research. By mandating specific enrollment objectives based on demographics such as race, ethnicity, sex, and age, this initiative plays a crucial role in improving the generalizability of research findings. It ensures that medical advancements benefit a broader population. This is evidenced by the increasing number of diversity-related postmarketing requirements and the ethical imperative for inclusive research practices. The DAP not only addresses current disparities but also underscores the importance of collaboration in advancing equitable healthcare solutions.
The FDA Diversity Action Plan signifies a crucial transformation in the realm of clinical research, targeting the rectification of historical disparities in participant representation. By mandating that sponsors establish specific enrollment objectives informed by demographic factors, this initiative not only bolsters the inclusivity of research studies but also aims to enhance the applicability of findings across varied populations.
As stakeholders navigate the complexities of implementing these requirements, pressing questions emerge:
The FDA Diversity Action Plan aims to significantly enhance the representation of historically underrepresented groups in research studies. This plan mandates that sponsors incorporate the FDA diversity action plan into their research protocols, outlining specific objectives for participant enrollment based on race, ethnicity, sex, and age. Importantly, the FDA diversity action plan requires that these enrollment objectives be detailed by demographic categories, fostering a systematic approach to diversity that addresses inequalities in research participation. By implementing this framework, the DAP aims to align with the FDA diversity action plan to ensure that study findings are applicable to a broader population, thereby improving the generalizability of research outcomes and enhancing patient results across diverse demographics.
Recent legislative changes underscore the necessity of inclusivity in medical research, reinforcing the FDA's commitment to promoting diversity. The DAP, known as the FDA diversity action plan, not only aims to rectify historical underrepresentation but also emphasizes the ethical and scientific importance of diverse participation in research studies. As articulated by the FDA, "Historically, underrepresented groups, including racial and ethnic minorities, women, older individuals, and those with comorbid conditions, have been significantly less involved in research studies." This statement highlights the urgent need for the FDA diversity action plan.
Furthermore, the relevance of the FDA diversity action plan is amplified by recent statistics showing that the number of diversity-related postmarketing requirements surged from one in 2018 to 14 in 2023. This trend reflects an increasing focus on diversity within medical trials. As the FDA continues to refine its recommendations, the FDA diversity action plan is set to play a pivotal role in shaping the future landscape of research, ensuring it includes the varied groups that will ultimately benefit from medical advancements. The comment period for the draft guidance concluded on September 26, 2024, illustrating the ongoing evolution of the FDA diversity action plan and demonstrating the FDA's responsiveness to stakeholder feedback.

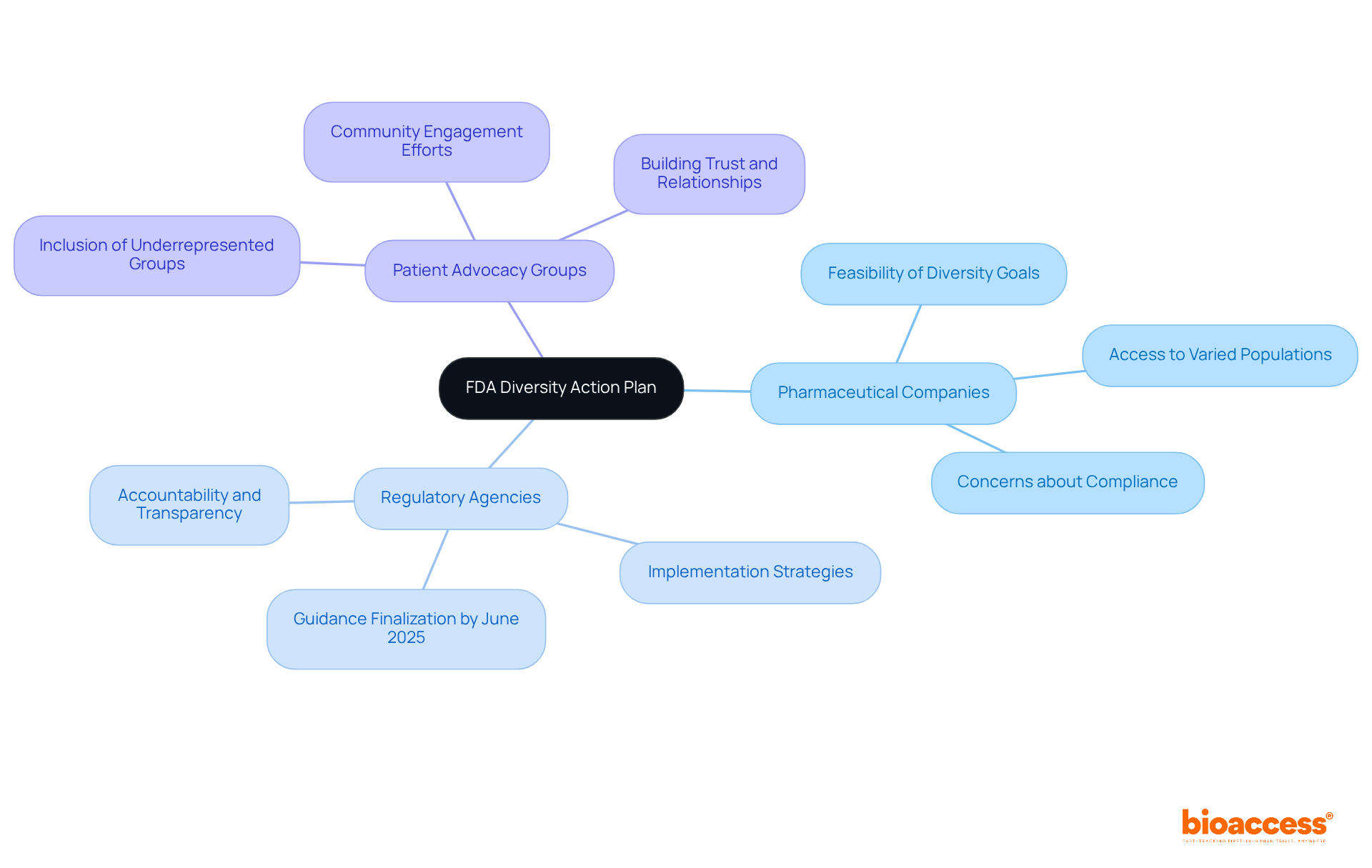
Stakeholders in medical research—including pharmaceutical companies, regulatory agencies, and patient advocacy groups—provide a diverse array of perspectives on the FDA Diversity Action Plan.
Pharmaceutical sponsors frequently voice concerns regarding the feasibility of achieving diversity goals, particularly in regions where access to varied populations is limited. This challenge is underscored by data revealing that in 2020, 75% of research participants were white, highlighting the pressing need for enhanced representation and its potential impact on the safety and effectiveness of medical products across all demographics.
Conversely, regulatory bodies emphasize the necessity of accountability and transparency within the FDA diversity action plan process, advocating for clear implementation strategies that ensure compliance and meaningful progress. With the FDA mandated to finalize its guidance by June 2025, this timeline is critical for stakeholders to monitor closely.
Patient advocacy organizations are integral to this dialogue, championing the inclusion of underrepresented groups to ensure studies accurately reflect the demographics of the general population. Community involvement efforts, for instance, have proven successful in building trust and fostering lasting relationships, which are essential for improving the recruitment and retention of diverse participants, as evidenced by case studies on community engagement in research.
Balancing these perspectives is crucial for the effective implementation of the FDA diversity action plan. Open dialogue and shared objectives among stakeholders can cultivate a more inclusive approach to medical research. As FDA Commissioner Robert M. Califf articulated, addressing the challenges of diversity in research studies transcends regulatory obligation; it is a moral imperative that can significantly enhance the safety and effectiveness of medical products for all groups.
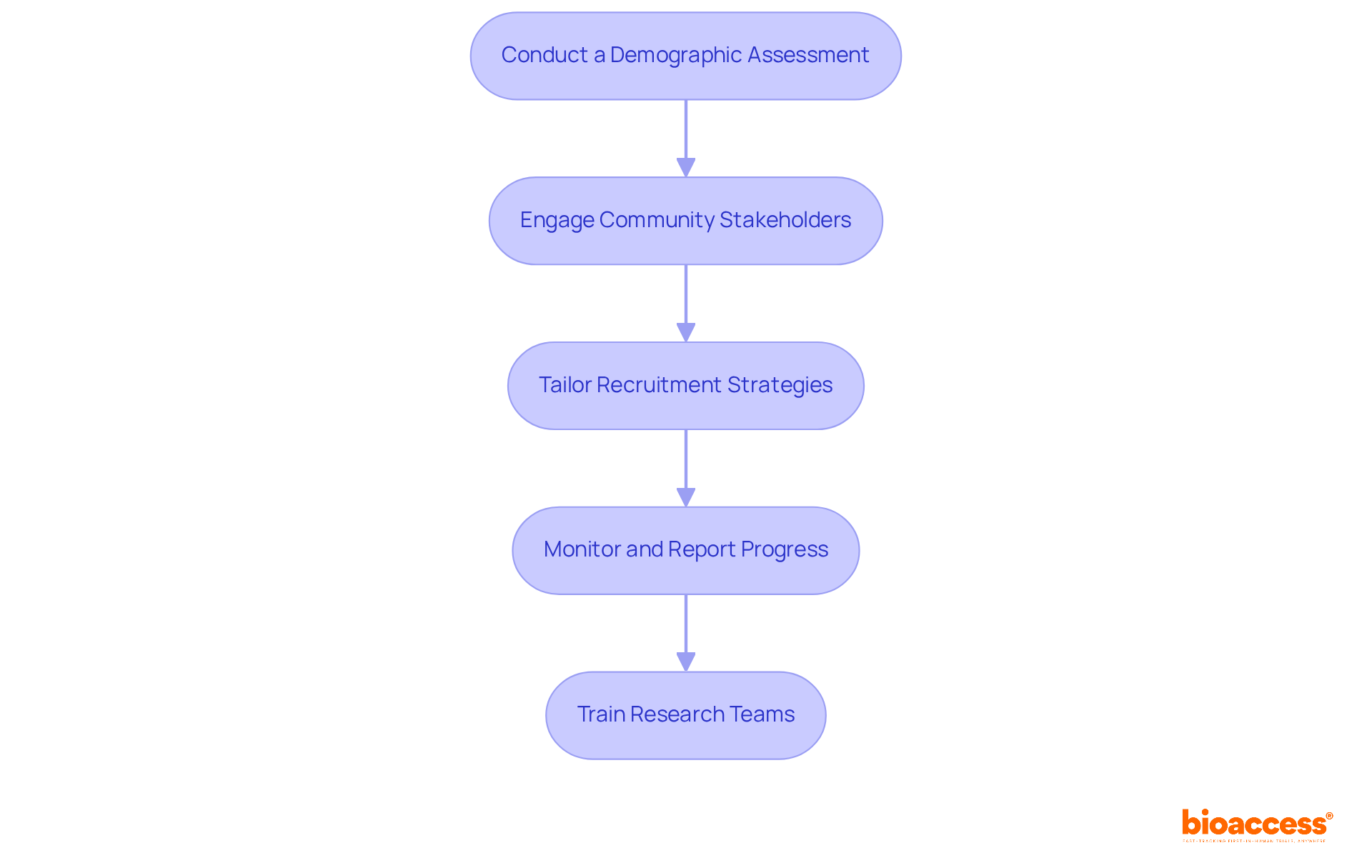
Sponsors must implement several essential strategies to comply with the FDA diversity action plan.
Conduct a Demographic Assessment: Before initiating a research study, it is crucial to carry out a comprehensive analysis of the target group's demographics to identify gaps in representation. This foundational step guides the establishment of realistic enrollment goals and tailored strategies.
Engage Community Stakeholders: Building alliances with community organizations and advocacy groups fosters trust and promotes involvement from marginalized groups. Effective community engagement can significantly enhance recruitment success rates.
Tailor Recruitment Strategies: Creating targeted recruitment campaigns that resonate with diverse communities is vital. Employing culturally relevant messaging and outreach methods ensures that recruitment efforts are not only inclusive but also effective in reaching potential participants.
Monitor and Report Progress: Developing metrics to track enrollment against the FDA diversity action plan goals is essential. Regular reporting to stakeholders fosters transparency and accountability, enabling timely adjustments to recruitment strategies as necessary.
Train Research Teams: Providing research personnel with education on cultural awareness and the essential role of diversity in medical studies enhances their ability to effectively engage with diverse populations. This training ultimately leads to improved recruitment outcomes.
The FDA Diversity Action Plan (DAP) has significant implications for global research trials, especially for sponsors conducting studies across various regions. As regulatory entities worldwide increasingly recognize the importance of diversity in medical research, the FDA diversity action plan serves as a model for similar initiatives in other countries. This evolution compels sponsors to embrace more inclusive practices, not only to meet FDA requirements but also to elevate the quality and applicability of their research findings.
Furthermore, the emphasis of the FDA diversity action plan on diverse participant enrollment can lead to improved patient outcomes and foster greater trust in research among underrepresented communities. Bioaccess supports this initiative through its comprehensive trial management services, which include:
By ensuring that studies are conducted in diverse environments and with varied participant demographics, Bioaccess assists sponsors in enhancing patient outcomes and building trust in clinical research among underrepresented communities. Consequently, sponsors may discover that embracing diversity not only satisfies regulatory requirements but also strengthens their market position and promotes innovation in drug and device development.
In conclusion, the FDA Diversity Action Plan stands as a pivotal initiative designed to enhance the representation of historically underrepresented groups in clinical research. By establishing specific enrollment objectives grounded in demographics, this plan aims to ensure that research findings resonate with a broader population, ultimately fostering improved health outcomes across diverse communities. This dedication to inclusivity not only addresses long-standing disparities but also aligns with ethical and scientific standards that prioritize the health of all populations.
Key insights from the article underscore the critical role of stakeholder engagement in the effective implementation of the FDA Diversity Action Plan. Pharmaceutical sponsors encounter challenges in meeting diversity goals, while regulatory bodies stress the necessity for accountability and transparency. Patient advocacy groups are indispensable in cultivating trust and community involvement, which are vital for enhancing the recruitment and retention of diverse participants. Employing strategies such as demographic assessments, community engagement, and tailored recruitment efforts is essential for both compliance and effectiveness.
Reflecting on the broader implications of the FDA Diversity Action Plan reveals that embracing diversity in clinical research transcends mere regulatory compliance; it embodies a moral imperative that enhances the safety and efficacy of medical products. As stakeholders collaboratively navigate the complexities of this initiative, the potential for improved patient outcomes and increased trust in research among underrepresented communities is substantial. By prioritizing diversity, the medical research landscape can evolve to better address the needs of all populations, ultimately leading to more equitable advancements in healthcare.
What is the FDA Diversity Action Plan?
The FDA Diversity Action Plan aims to enhance the representation of historically underrepresented groups in research studies by mandating sponsors to incorporate specific enrollment objectives based on race, ethnicity, sex, and age into their research protocols.
Why is the FDA Diversity Action Plan important?
It addresses inequalities in research participation, ensuring that study findings are applicable to a broader population, which improves the generalizability of research outcomes and enhances patient results across diverse demographics.
What groups are considered historically underrepresented in research studies?
Historically underrepresented groups include racial and ethnic minorities, women, older individuals, and those with comorbid conditions.
How has the focus on diversity in medical trials changed recently?
The number of diversity-related postmarketing requirements surged from one in 2018 to 14 in 2023, indicating an increasing emphasis on diversity within medical trials.
What does the FDA require regarding demographic categories in research?
The FDA requires that enrollment objectives in research studies be detailed by demographic categories to foster a systematic approach to diversity.
When did the comment period for the draft guidance of the FDA Diversity Action Plan conclude?
The comment period for the draft guidance concluded on September 26, 2024.
How does the FDA Diversity Action Plan align with recent legislative changes?
Recent legislative changes underscore the necessity of inclusivity in medical research, reinforcing the FDA's commitment to promoting diversity through the Diversity Action Plan.