


To maximize success in the Latin American market for clinical trials, sponsors must leverage the region's efficient regulatory processes, engage local stakeholders, and implement effective patient recruitment strategies. This approach is crucial for enhancing trial efficiency and patient access to new treatments.
By utilizing the following strategies, sponsors can significantly improve outcomes:
These strategies not only streamline processes but also ensure that patients receive timely access to innovative therapies, addressing the critical need for effective clinical research in the region.
Latin America is emerging as a pivotal hub for clinical trials, showcasing efficient regulatory processes and a diverse patient population that can significantly enhance research outcomes. By leveraging the region’s unique advantages—such as fast-track approval pathways and substantial tax incentives—sponsors can streamline their clinical studies and expedite patient access to innovative therapies.
However, navigating the complexities of local market dynamics, cultural nuances, and effective stakeholder collaborations presents a challenge. How can clinical trial sponsors capitalize on these opportunities while overcoming potential obstacles to maximize their success in this promising market?
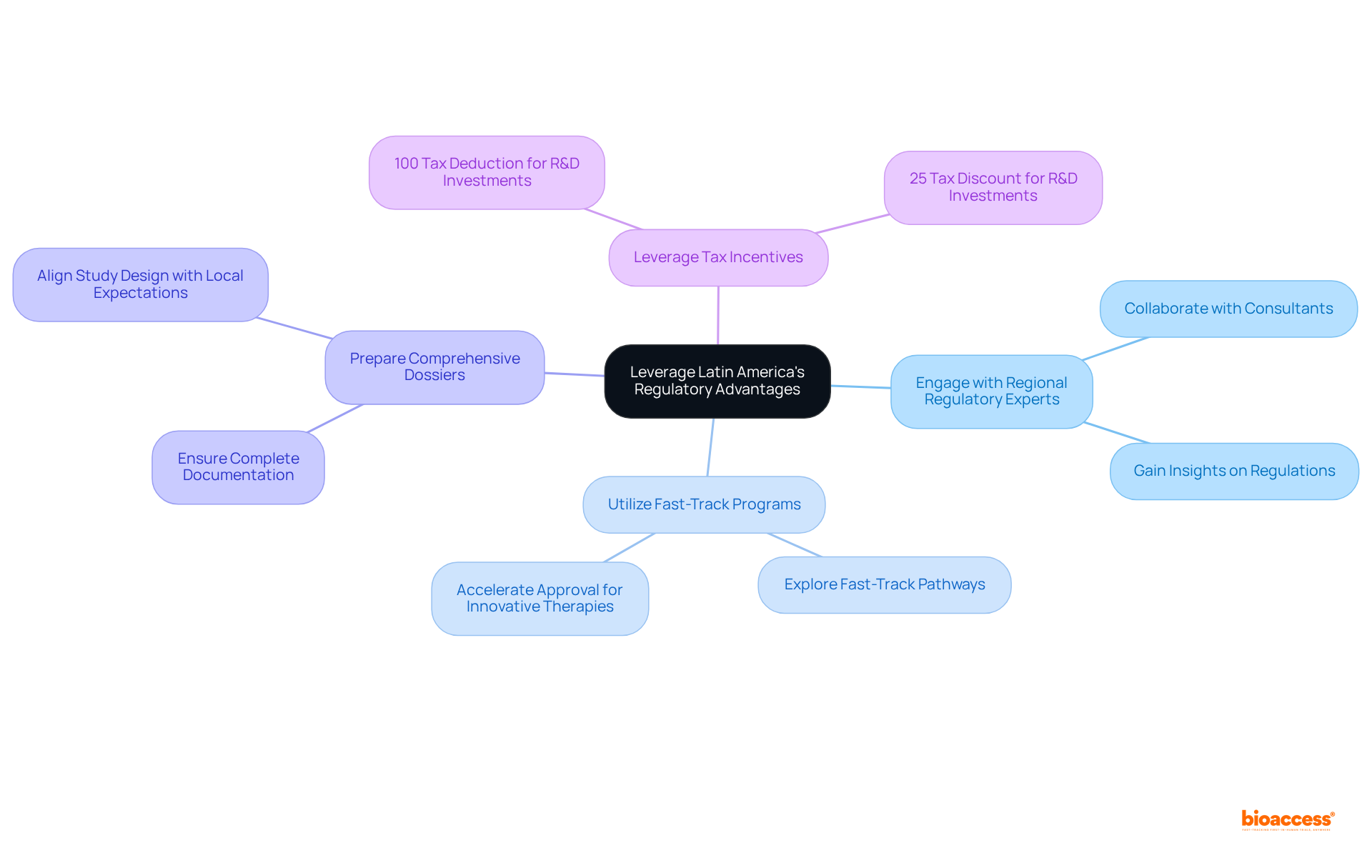
Latin America is recognized for its efficient regulatory processes, particularly in Brazil and Colombia, where ethical approvals can be achieved in as little as 4-6 weeks. In Colombia, the total IRB/EC and MoH (INVIMA) review lasts about 90-120 days, making it an appealing choice for research sponsors. To capitalize on these advantages, clinical trial sponsors should consider the following strategies:
By strategically utilizing these regulatory benefits, sponsors can significantly decrease the time and expenses associated with research studies in the Latin American market, ultimately resulting in quicker patient access to new treatments.
A recent clinical trial for a novel biopharmaceutical in Colombia was approved in just four weeks, enabling the sponsor to initiate patient recruitment ahead of schedule. This case exemplifies the potential benefits of effectively understanding and utilizing local regulatory advantages. Furthermore, GlobalCare Clinical Trials and bioaccess® achieved a 95% retention rate, showcasing the effectiveness of collaborations in enhancing participant engagement and retention.
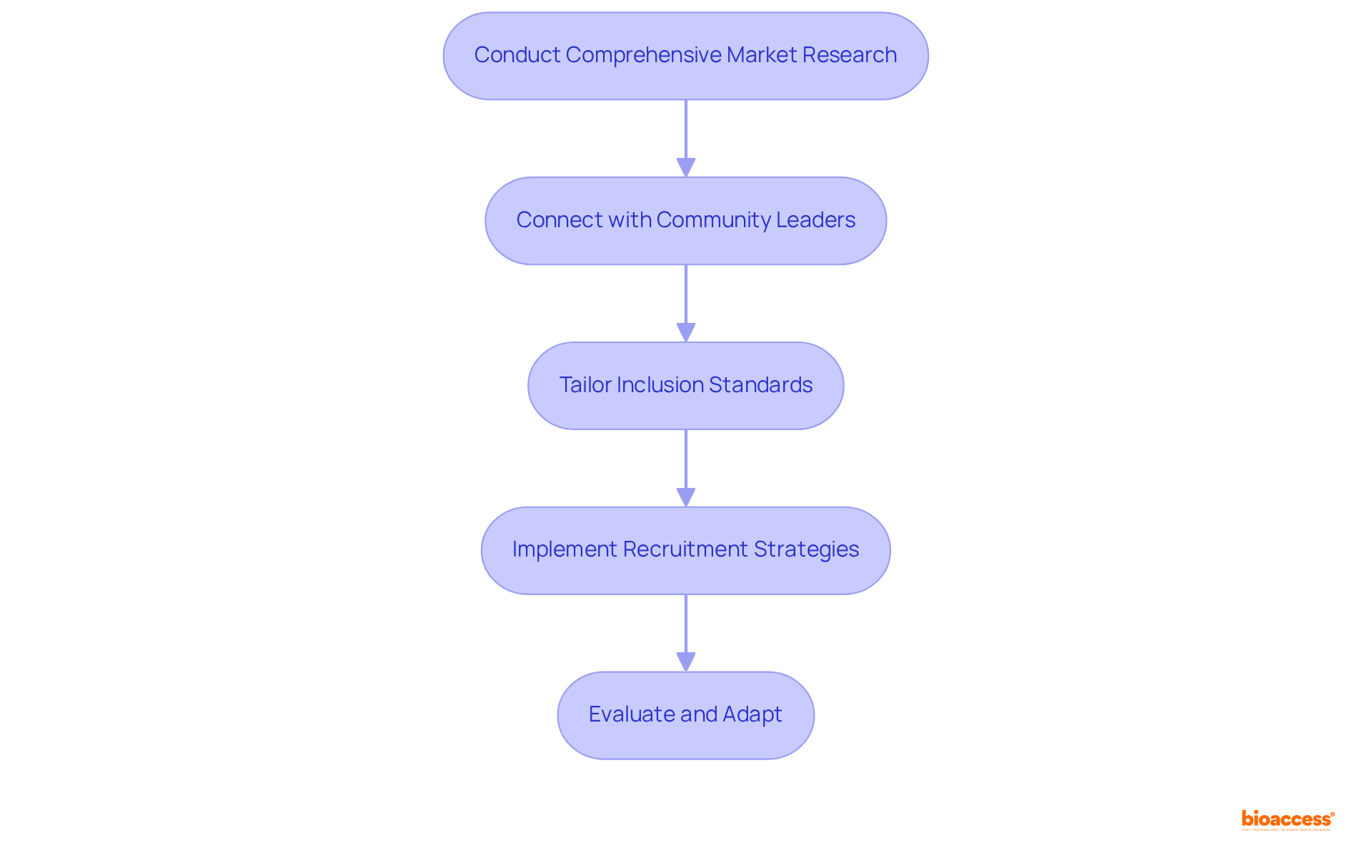
Latin America hosts a diverse population with varying health profiles that can significantly influence clinical study outcomes. To effectively navigate this landscape, sponsors must take strategic actions:
Conduct Comprehensive Market Research: Understanding the regional healthcare environment, including prevalent illnesses and treatment gaps, is crucial for guiding trial design and patient selection strategies. For instance, ReGelTec's Early Feasibility Study on HYDRAFIL™ for chronic low back pain in Colombia underscores the importance of tailoring approaches to community health issues, as evidenced by the successful treatment of eleven patients with degenerative disc disease.
Connect with Community Leaders: Building relationships with local healthcare providers and community figures can cultivate trust and enhance recruitment efforts, particularly in underserved populations. Collaborating with nearby clinics and community health workers can significantly improve outreach and engagement.
Tailor Inclusion Standards: Adjusting inclusion and exclusion criteria to accurately reflect the regional patient population can enhance participant engagement and ensure that the study results are pertinent to the intended demographic. This strategy was exemplified in the aforementioned study, where the unique needs of the Colombian patient population were thoughtfully considered.
Real-World Example: A biopharma company conducting a trial for a diabetes medication in Mexico adapted its recruitment strategy by partnering with local clinics and community health workers. This approach led to a remarkable 50% increase in patient enrollment compared to traditional methods, highlighting the critical importance of understanding regional market dynamics.
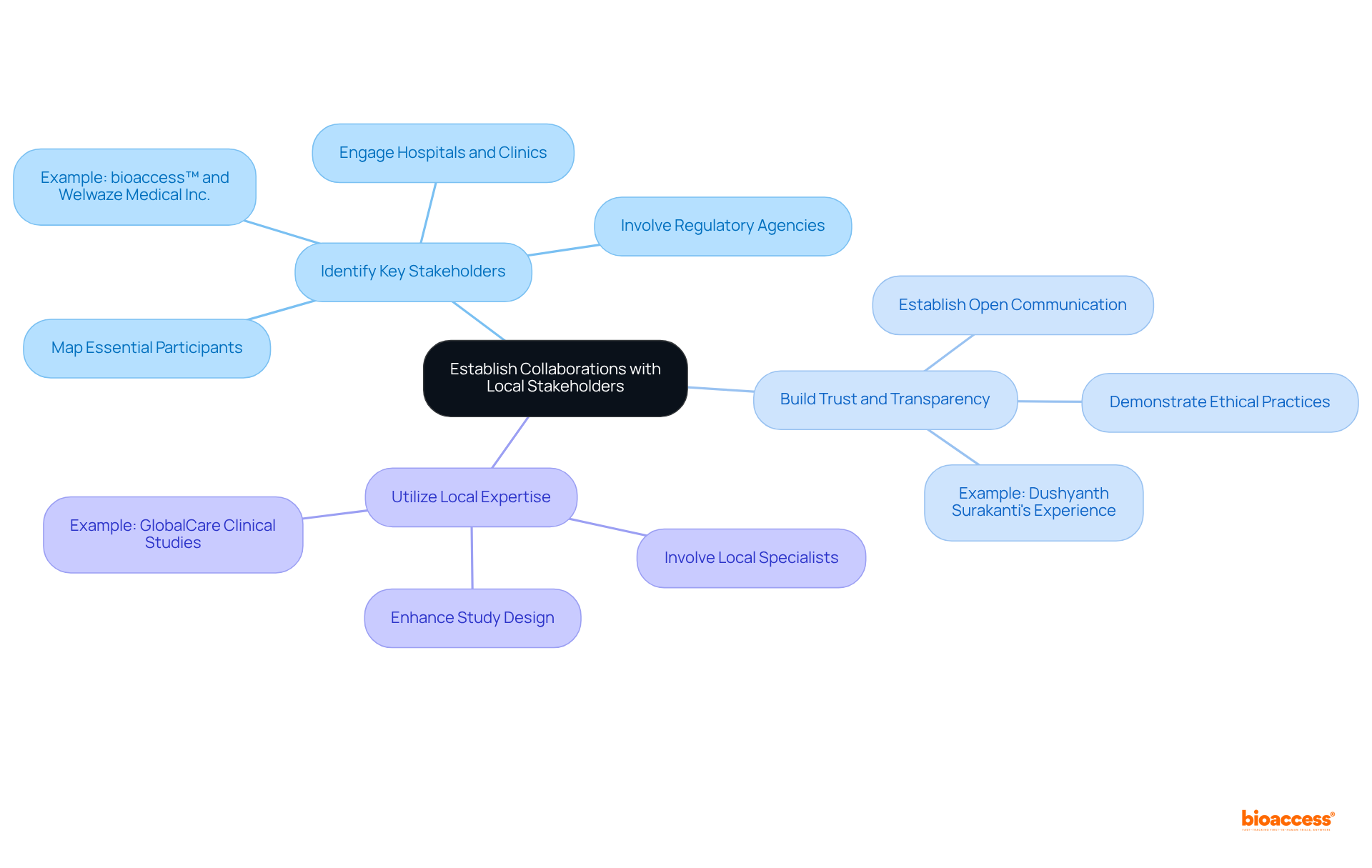
Successful clinical trials in the Latin American market often hinge on the strength of regional collaborations. To foster these partnerships, sponsors should:
Identify Key Stakeholders: Mapping out essential participants in the regional healthcare ecosystem—including hospitals, clinics, and regulatory agencies—enables sponsors to engage effectively. For instance, bioaccess™ has successfully partnered with Welwaze Medical Inc. to facilitate the launch of the Celbrea® medical device in Colombia, underscoring the significance of involving regional entities.
Build Trust and Transparency: Establishing open lines of communication and demonstrating a commitment to ethical practices fosters trust among stakeholders, which is crucial for collaboration. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, highlighted his positive experience with bioaccess® during its initial human study in Colombia, emphasizing the vital role of trust in these partnerships.
Utilize Local Expertise: Involving local specialists who understand the cultural and regulatory environment provides essential insights that enhance study design and implementation. For example, GlobalCare Clinical Studies collaborated with bioaccess™ to improve ambulatory services for research in Colombia, achieving over a 50% decrease in recruitment duration and 95% retention rates. bioaccess™ offers critical services such as regulatory approval, research site activation, and study data management, which are indispensable for successful partnerships.
Real-World Example:
A Medtech company partnered with a local university in Brazil to conduct a clinical trial for a new medical device. This collaboration not only granted access to a diverse patient group but also facilitated smoother regulatory interactions, culminating in the successful conclusion of the study ahead of schedule. bioaccess™ played a pivotal role in navigating these regulatory processes, ensuring a streamlined approach.
Statistics indicate that 76.8% of studies in the region had a Latin American writer as the primary author, highlighting the importance of regional participation in research. Furthermore, the Latin American market for trials is projected to expand at a CAGR of 8-12%, underscoring the growing significance of strategic alliances in this evolving landscape. By prioritizing local collaborations, companies can adeptly navigate the complexities of the healthcare ecosystem, ultimately leading to more successful clinical outcomes.
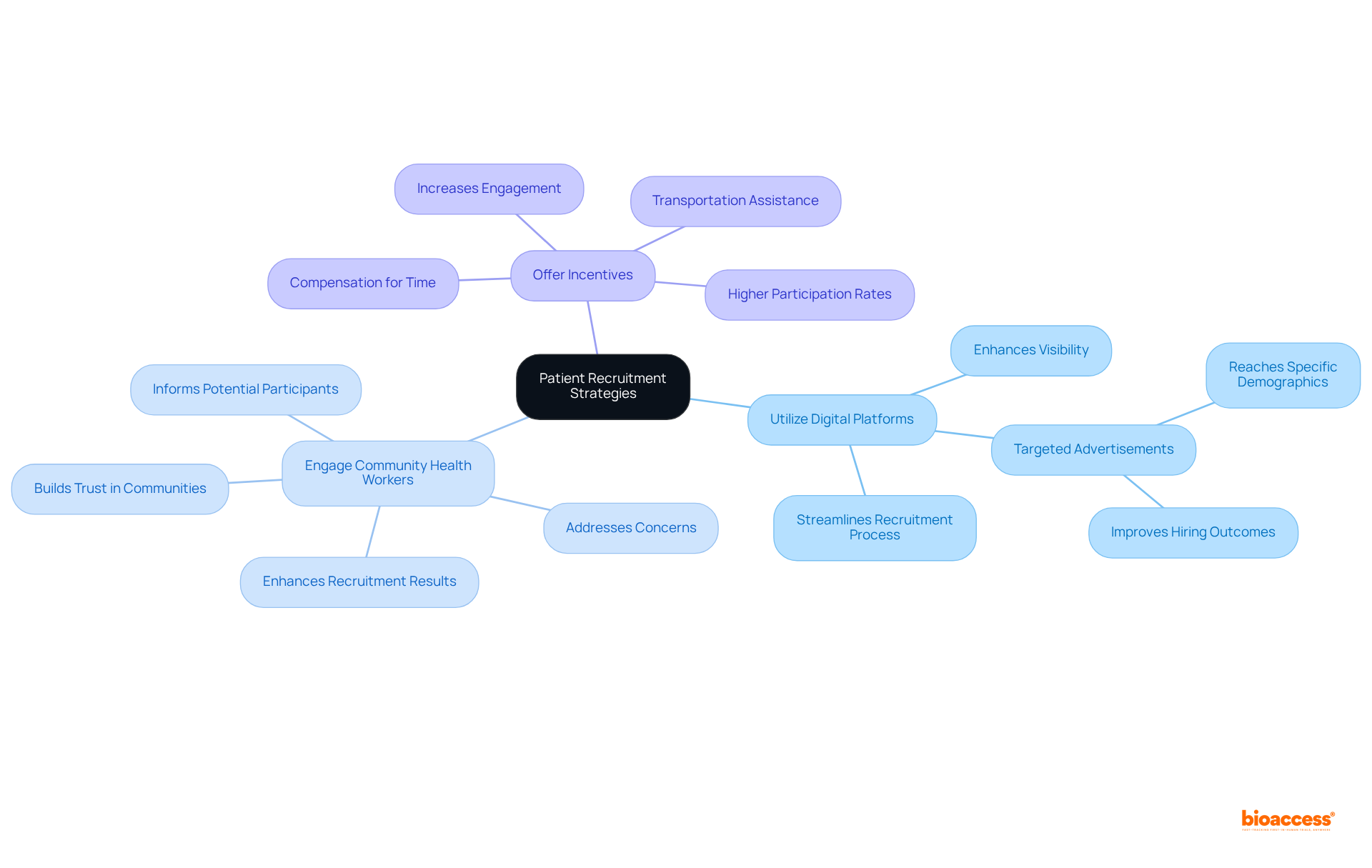
To optimize patient recruitment in Latin America, sponsors should consider several effective strategies:
Utilize Digital Platforms: Leveraging social media platforms such as Facebook, Instagram, and online health forums is crucial for reaching potential participants, particularly younger demographics who are more likely to engage online. Digital tools not only enhance visibility but also streamline the recruitment process, making it more accessible for diverse populations. For instance, targeted advertisements on these platforms can effectively reach specific demographics, improving hiring outcomes.
Engage Community Health Workers: Partnering with regional health workers can significantly improve outreach efforts. These individuals often have established trust within their communities and can effectively inform potential participants about the study, addressing any concerns and fostering a supportive environment for enrollment. Studies show that community involvement methods can result in enhanced recruitment results, as these individuals can connect the divide between research studies and nearby populations.
Offer Incentives: Providing incentives for participation, such as transportation assistance or compensation for time, can improve enrollment rates, particularly in lower-income areas. This method recognizes the obstacles encountered by prospective participants and promotes increased engagement in clinical studies. Successful examples in the Latin American market have shown that offering such incentives can lead to higher participation rates and better retention.
A recent trial for a new cancer treatment in Argentina effectively utilized a combination of social media campaigns and partnerships with local health workers, resulting in a remarkable 70% increase in patient enrollment compared to previous trials. This case highlights the effectiveness of customized hiring strategies in engaging varied populations and achieving hiring objectives. Additionally, collaborations like that between GlobalCare Clinical Trials and bioaccess™ have demonstrated significant reductions in enrollment time by over 50% and retention rates of 95%, further illustrating the potential of strategic partnerships in enhancing recruitment efforts. The support from Colombia's Minister of Health for initiatives like these positions Barranquilla as a leading destination for clinical trials in the Latin American market, paving the way for future growth and success.
Maximizing success in the Latin American market for clinical trials hinges on understanding and leveraging the region's unique regulatory advantages, diverse patient populations, and the importance of local collaborations. By strategically engaging with regulatory experts, utilizing fast-track programs, and preparing comprehensive submissions, sponsors can significantly expedite the approval process and enhance patient access to innovative therapies.
This article highlights several key strategies:
Real-world examples underscore the tangible benefits of these approaches, demonstrating that successful clinical trials in Latin America are achievable through careful planning and execution.
As the Latin American clinical trials market continues to grow, embracing these best practices becomes essential for sponsors aiming to navigate the complexities of the region. By prioritizing local partnerships and understanding the unique dynamics at play, stakeholders can not only improve trial outcomes but also contribute to the advancement of medical research that is culturally and regionally relevant. Engaging with this vibrant market presents an opportunity to enhance global health outcomes while fostering innovation and collaboration in the clinical research landscape.
What are the regulatory advantages of conducting clinical trials in Latin America?
Latin America, particularly Brazil and Colombia, is known for efficient regulatory processes, with ethical approvals achievable in as little as 4-6 weeks in Brazil and a total IRB/EC and MoH (INVIMA) review lasting about 90-120 days in Colombia.
How can clinical trial sponsors capitalize on Latin America's regulatory advantages?
Sponsors can engage with regional regulatory experts, utilize fast-track programs, prepare comprehensive dossiers, and leverage tax incentives to enhance their research processes and approvals.
What role do regional regulatory experts play in the clinical trial process?
Collaborating with regional regulatory consultants provides insights into the specific nuances of each nation's regulations, ensuring compliance and facilitating a smoother approval process.
What are fast-track programs in Latin America?
Fast-track programs are expedited approval pathways offered by numerous Latin American countries for innovative therapies, allowing sponsors to accelerate their clinical trials.
Why is it important to prepare comprehensive regulatory dossiers?
Thoroughly prepared regulatory submissions that adhere to regional requirements are essential to prevent delays and ensure that all necessary documentation is complete and aligned with local expectations.
What tax incentives does Colombia offer for research and development?
Colombia provides substantial R&D tax incentives, including a 100% tax deduction for investments in science, technology, and innovation projects, along with additional financial incentives.
How can leveraging regulatory advantages impact patient access to treatments?
By strategically utilizing regulatory benefits, sponsors can significantly decrease the time and costs associated with research studies, resulting in quicker patient access to new treatments.
Can you provide an example of a successful clinical trial in Colombia?
A recent clinical trial for a novel biopharmaceutical in Colombia was approved in just four weeks, allowing the sponsor to begin patient recruitment ahead of schedule, demonstrating the potential benefits of understanding local regulatory advantages.
What was the retention rate achieved by GlobalCare Clinical Trials and bioaccess®?
GlobalCare Clinical Trials and bioaccess® achieved a 95% retention rate, showcasing the effectiveness of collaborations in enhancing participant engagement and retention.