


Turnkey project services in clinical research are pivotal for maximizing success by providing a comprehensive, single-contractor approach that effectively manages all project aspects, from planning to execution. This model ensures regulatory compliance and operational efficiency, making it highly relevant in today’s clinical research landscape.
The benefits of this approach are significant:
These collectively enhance trial capabilities and improve overall outcomes for organizations within the Medtech, Biopharma, and Radiopharma sectors. As clinical research continues to evolve, understanding and leveraging these advantages is essential for overcoming key challenges and achieving superior results.
Turnkey project services in clinical research signify a transformative approach that consolidates all project elements under a single contractor, optimizing operations and enhancing efficiency. Organizations in the Medtech, Biopharma, and Radiopharma sectors are poised to gain significantly from this model, which not only simplifies management but also accelerates timelines and reduces costs.
As the landscape of clinical research evolves, pertinent questions emerge:
Turnkey projects services in medical research represent a comprehensive strategy where a single contractor manages all aspects of a project from initiation to conclusion. This model necessitates meticulous planning, execution, and delivery of trials, ensuring compliance with all requirements and readiness for swift operation upon transfer. By assuming complete responsibility for the project, the contractor significantly minimizes the client's involvement in daily operations, allowing them to focus on strategic decision-making.
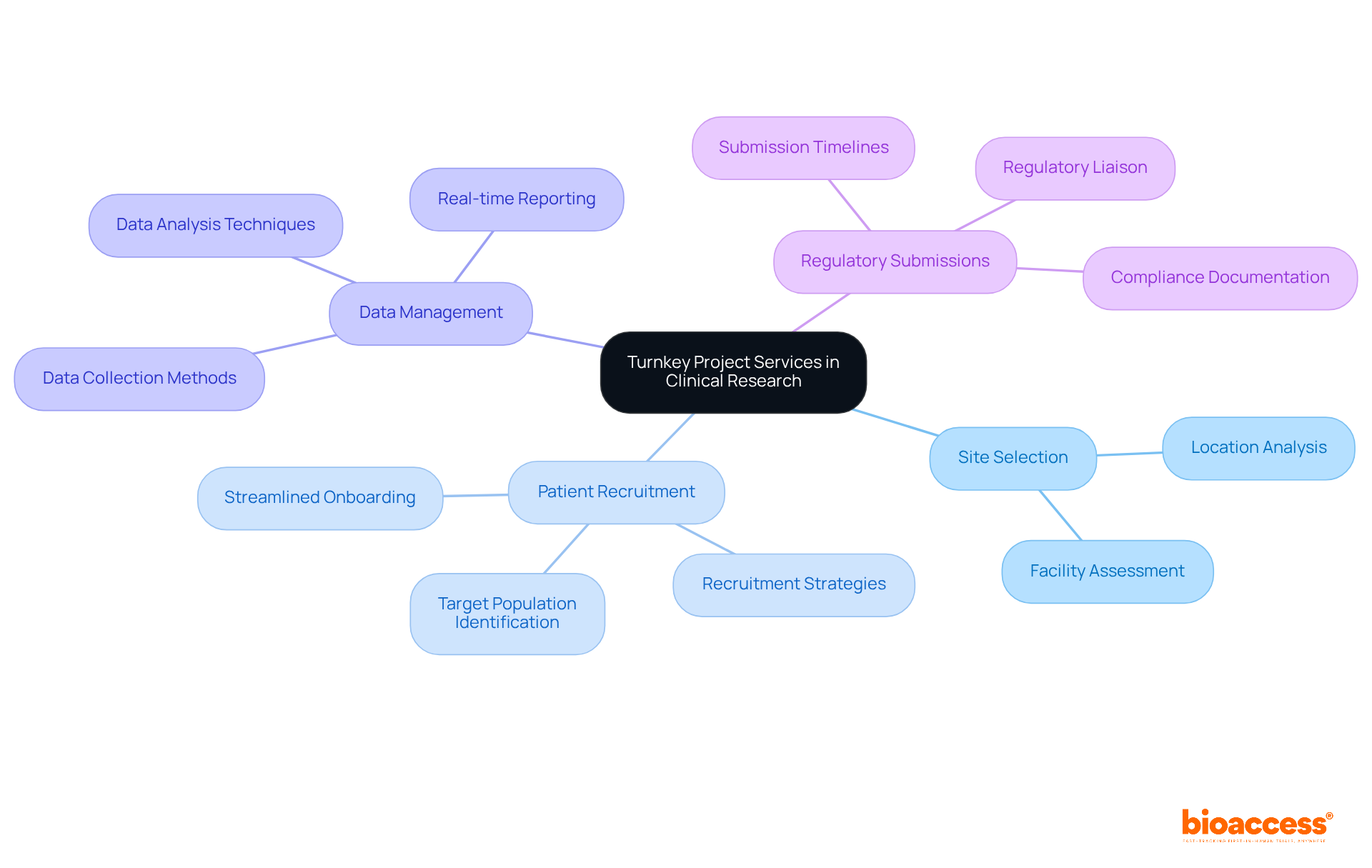
This method proves particularly advantageous in medical research, where strict adherence to timelines and regulatory compliance is paramount. Turnkey projects services provide a streamlined solution that integrates critical functions such as:
This integration not only enhances the overall efficiency of medical trials but also accelerates the journey to successful outcomes, establishing it as a preferred choice for numerous organizations within the Medtech, Biopharma, and Radiopharma sectors.
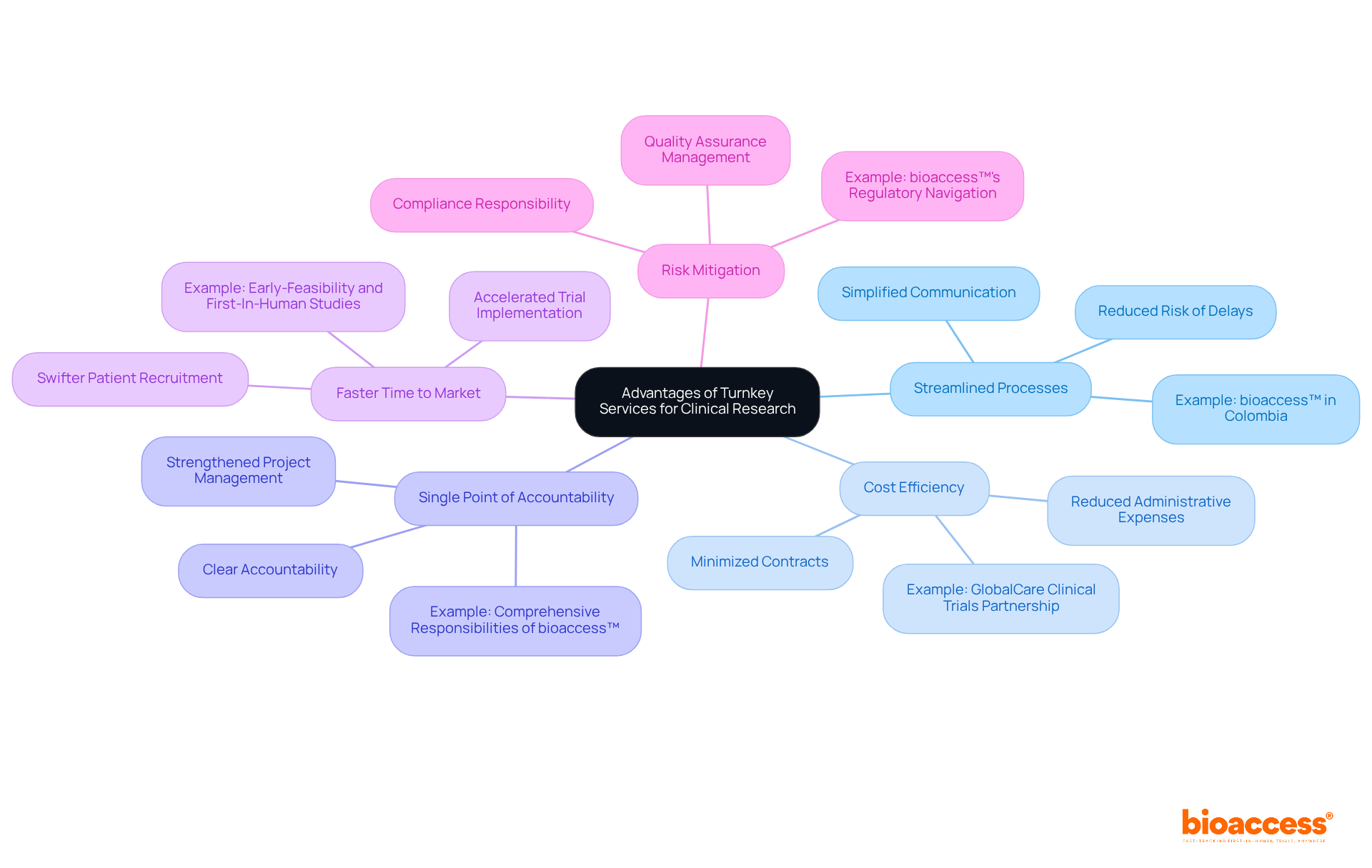
Turnkey projects services in clinical research offer a multitude of compelling advantages that are crucial for organizations in the Medtech, Biopharma, and Radiopharma sectors.
Streamlined Processes: Consolidating all components under a single contractor simplifies communication and enhances efficiency, significantly reducing the risk of delays. This approach is exemplified by bioaccess™, which has successfully facilitated the entry of innovative medical devices like Celbrea® into the Colombian market, showcasing their ability to manage complex regulatory landscapes efficiently.
Cost efficiency can be realized through turnkey projects services, which minimize the need for multiple contracts and reduce administrative expenses, resulting in substantial cost savings. Partnerships, such as that of GlobalCare Clinical Trials with bioaccess™, have resulted in over a 50% reduction in recruitment time, further enhancing cost-effectiveness.
Single Point of Accountability: With one contractor overseeing the entire project, accountability is clear, which strengthens project management and oversight. Bioaccess™ exemplifies this by taking on comprehensive responsibilities in trial management, ensuring that all aspects are aligned and accountable.
Faster Time to Market: This integrated method accelerates the implementation of trials, enabling swifter patient recruitment and data gathering, ultimately leading to quicker regulatory approvals. Bioaccess™ has demonstrated its capability in expediting medical device clinical studies across Latin America, including Early-Feasibility and First-In-Human studies.
Risk Mitigation: Turnkey projects services effectively mitigate risks associated with project management, as the contractor assumes responsibility for compliance and quality assurance throughout the project lifecycle. Bioaccess™'s extensive experience in navigating regulatory requirements further enhances risk management for their clients.
These advantages establish turnkey projects services as a strategic option for firms seeking to enhance their trial capabilities and improve their overall operational efficiency.
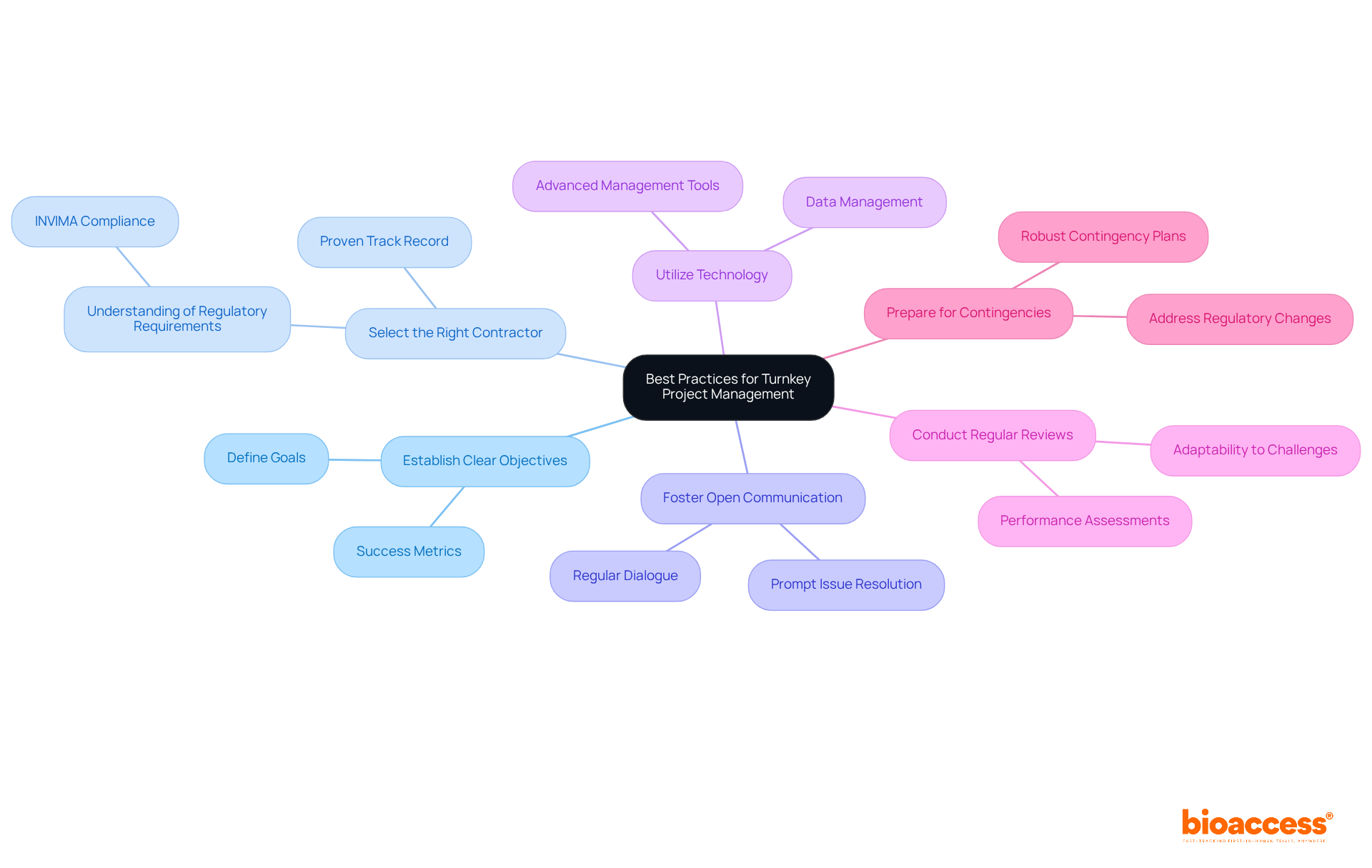
To maximize the effectiveness of turnkey projects services in clinical research, organizations must adopt essential best practices that drive success.
Establish Clear Objectives: Clearly defining goals and success metrics from the outset ensures alignment among all stakeholders. This alignment is crucial for maintaining focus and direction throughout the project.
Select the Right Contractor: Choosing a contractor with a proven track record in clinical research and a comprehensive understanding of regulatory requirements—such as those outlined by INVIMA, Colombia's National Food and Drug Surveillance Institute—guarantees adherence and quality throughout the lifecycle. This is particularly important in areas like feasibility studies, site selection, and compliance reviews.
Foster Open Communication: Maintaining regular dialogue with the contractor and stakeholders allows for the prompt addressing of any issues that arise. This proactive approach keeps the endeavor on track and minimizes potential delays.
Utilize Technology: Leveraging advanced management tools and software enhances collaboration, tracks progress, and manages data effectively. This technological integration significantly streamlines operations and improves overall efficiency.
Conduct Regular Reviews: Implementing periodic performance reviews to assess progress against objectives enables organizations to make prompt modifications to strategies. This adaptability ensures that the initiative remains aligned with its goals, even in the face of challenges such as scope creep or miscommunication.
Prepare for Contingencies: Developing robust contingency plans to address potential challenges—such as regulatory changes or recruitment difficulties—ensures that the initiative can adapt to unforeseen circumstances. This proactive strategy safeguards the success of the project.
By adhering to these best practices, organizations can significantly enhance the success rates of their turnkey projects services, thereby achieving clinical research objectives more efficiently and effectively. Incorporating tailored solutions from bioaccess can lead to improved patient outcomes and operational excellence.
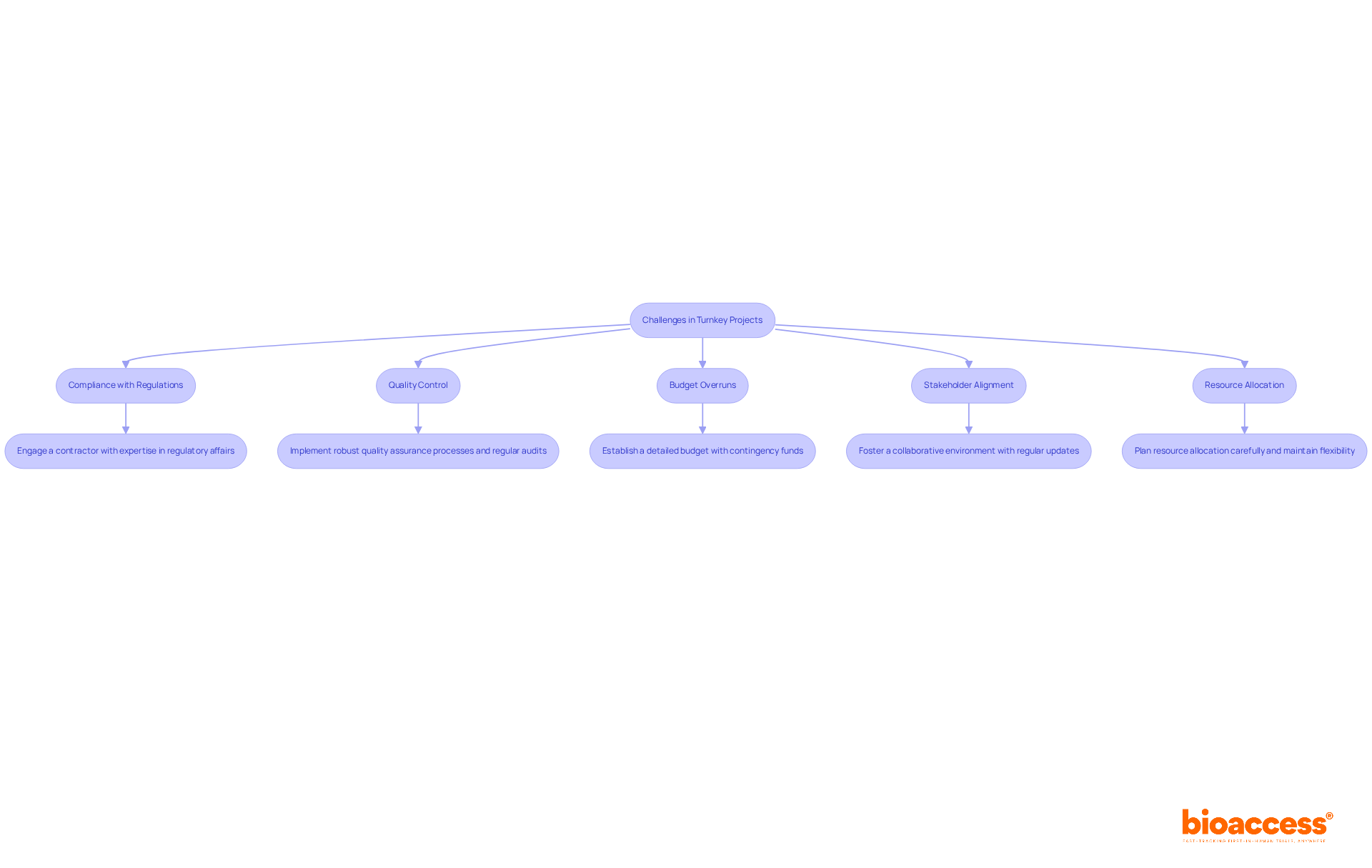
Turnkey projects provide a range of benefits, yet they also pose significant challenges that must be effectively addressed:
By proactively addressing these challenges with effective solutions, organizations can enhance the success of their turnkey projects services and achieve their clinical research goals.
Turnkey project services in clinical research represent a transformative approach that streamlines operations, enhances accountability, and accelerates the path to successful outcomes. By consolidating all project components under a single contractor, organizations can significantly reduce administrative burdens and focus on strategic objectives. This ultimately leads to improved efficiency and effectiveness in clinical trials.
The advantages of turnkey services are manifold, including:
Best practices such as establishing clear objectives, selecting the right contractor, fostering open communication, and preparing for contingencies are essential strategies for maximizing the success of these projects. Moreover, addressing challenges like regulatory compliance and stakeholder alignment with proactive solutions ensures that organizations can navigate potential obstacles effectively.
The significance of embracing turnkey project services in clinical research cannot be overstated. As the landscape of clinical trials continues to evolve, organizations are encouraged to adopt this comprehensive approach to enhance their operational capabilities and drive innovation in patient outcomes. By leveraging the insights and strategies outlined, stakeholders can position themselves for success in a competitive and rapidly changing environment.
What are turnkey project services in clinical research?
Turnkey project services in clinical research refer to a comprehensive approach where a single contractor manages all aspects of a project from initiation to conclusion, ensuring meticulous planning, execution, and delivery of trials.
What are the benefits of using turnkey project services?
The benefits include reduced client involvement in daily operations, allowing them to focus on strategic decision-making, and ensuring compliance with timelines and regulatory requirements.
What critical functions are integrated into turnkey project services?
Turnkey project services integrate critical functions such as site selection, patient recruitment, data management, and regulatory submissions.
Why are turnkey project services particularly advantageous in medical research?
They are advantageous because they enhance overall efficiency, accelerate the journey to successful outcomes, and ensure strict adherence to timelines and regulatory compliance.
Which sectors commonly utilize turnkey project services?
Turnkey project services are preferred by organizations within the Medtech, Biopharma, and Radiopharma sectors.