


Navigating the intricate landscape of biologic drug approval in Macedonia demands a thorough understanding of the regulatory framework that governs this essential process.
With the Macedonian Agency for Medicines and Medical Equipment leading the charge, stakeholders must familiarize themselves with crucial laws and guidelines to ensure compliance and streamline submissions.
As the regulatory environment continues to evolve, how can developers effectively navigate potential roadblocks and accelerate their approval timelines?
This guide presents a comprehensive roadmap for mastering the biologic drug approval procedure in Macedonia, equipping readers with the knowledge and strategies necessary to enhance their chances of success.
To effectively manage the biologic drug approval procedure in Macedonia, it is essential to understand the regulatory structure that oversees biologics. The Macedonian Agency for Medicines and Medical Equipment is the primary organization supervising this process. Familiarize yourself with the following key regulations:
By comprehending these regulatory aspects, you will be better equipped to navigate the biologic drug approval procedure in Macedonia efficiently, ensuring compliance and facilitating successful biologic drug endorsements.
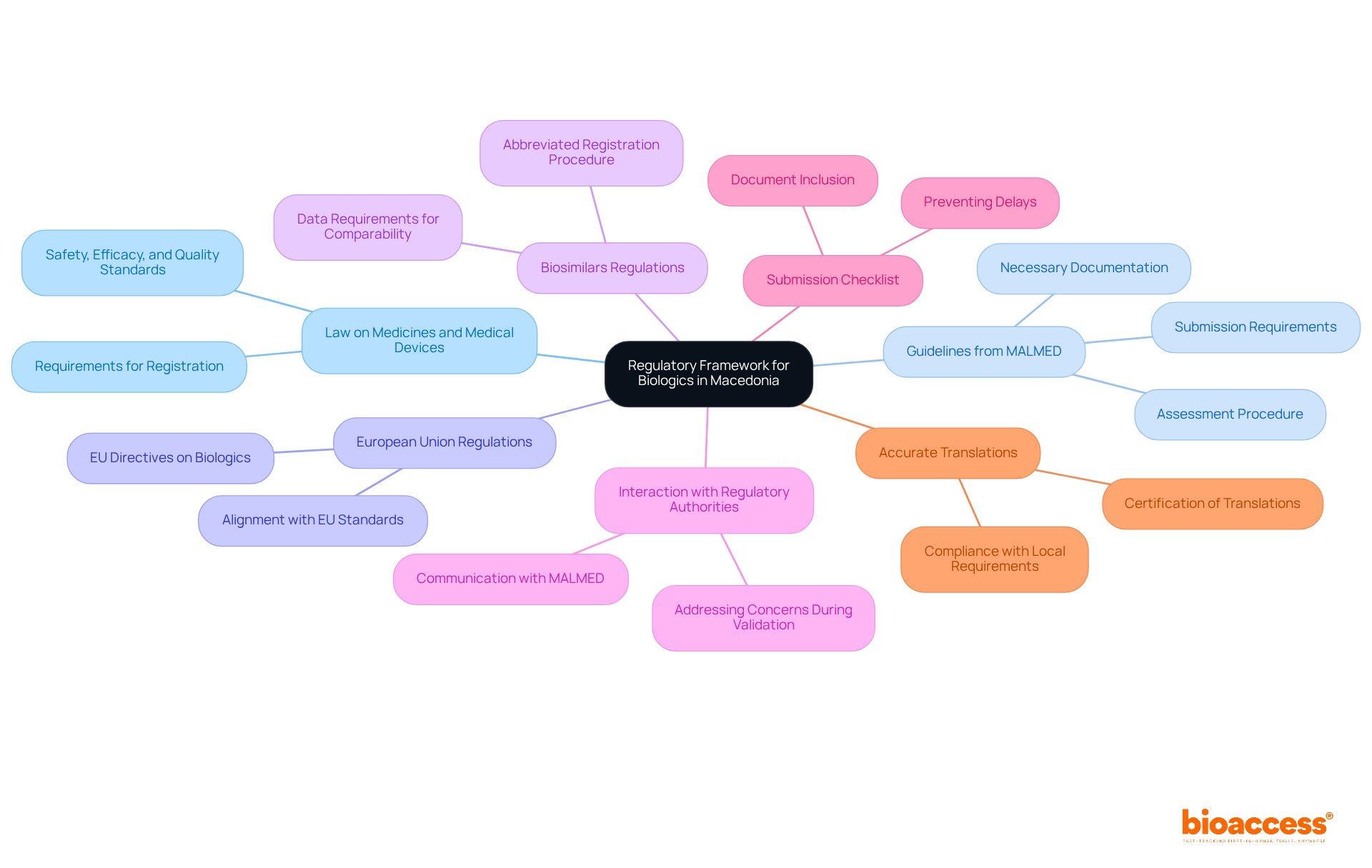
To effectively navigate the approval process for biologics in Macedonia, follow these structured steps:
Pre-Submission Preparation: Compile all essential documentation, including preclinical data, clinical trial protocols, and manufacturing details. Verify that your product meets the eligibility criteria established by the Macedonian Agency for Medicines and Medical Devices. Ensure compliance with Good Manufacturing Practices (GMP) and Good Clinical Practices (GCP) to maintain product quality and avoid penalties.
Submit Application to the Organization: Carefully prepare and submit your application to the organization, ensuring that all required forms and supporting documents are included as per their guidelines. This includes proof of payment for submission fees.
Review Process: After submission, MALMED will conduct a comprehensive review of your application. It is crucial to respond swiftly to any inquiries or requests for additional information to avoid delays in the review process. Bioaccess can assist in this stage by providing project management services to ensure timely responses and adherence to all requirements.
Consent Notification: Once consent is granted, you will receive a message from the organization outlining any conditions or post-consent requirements that must be met.
Market Authorization: After obtaining consent, you can commence the market authorization procedure, ensuring adherence to ongoing reporting and monitoring requirements. Bioaccess offers expertise in managing these obligations effectively.
Stay Informed: Regularly review resources like the official MALMED website or industry publications to stay updated on regulatory changes, including the upcoming 2025 guidelines, which are crucial for successful submissions.
By carefully adhering to these steps, you can simplify the biologic drug approval procedure in Macedonia and significantly enhance your chances of a successful submission. Bioaccess provides comprehensive clinical trial management services, including expertise in feasibility studies and site selection for clinical trial protocols, which can further optimize your application and potentially expedite clinical trials by up to 40%.
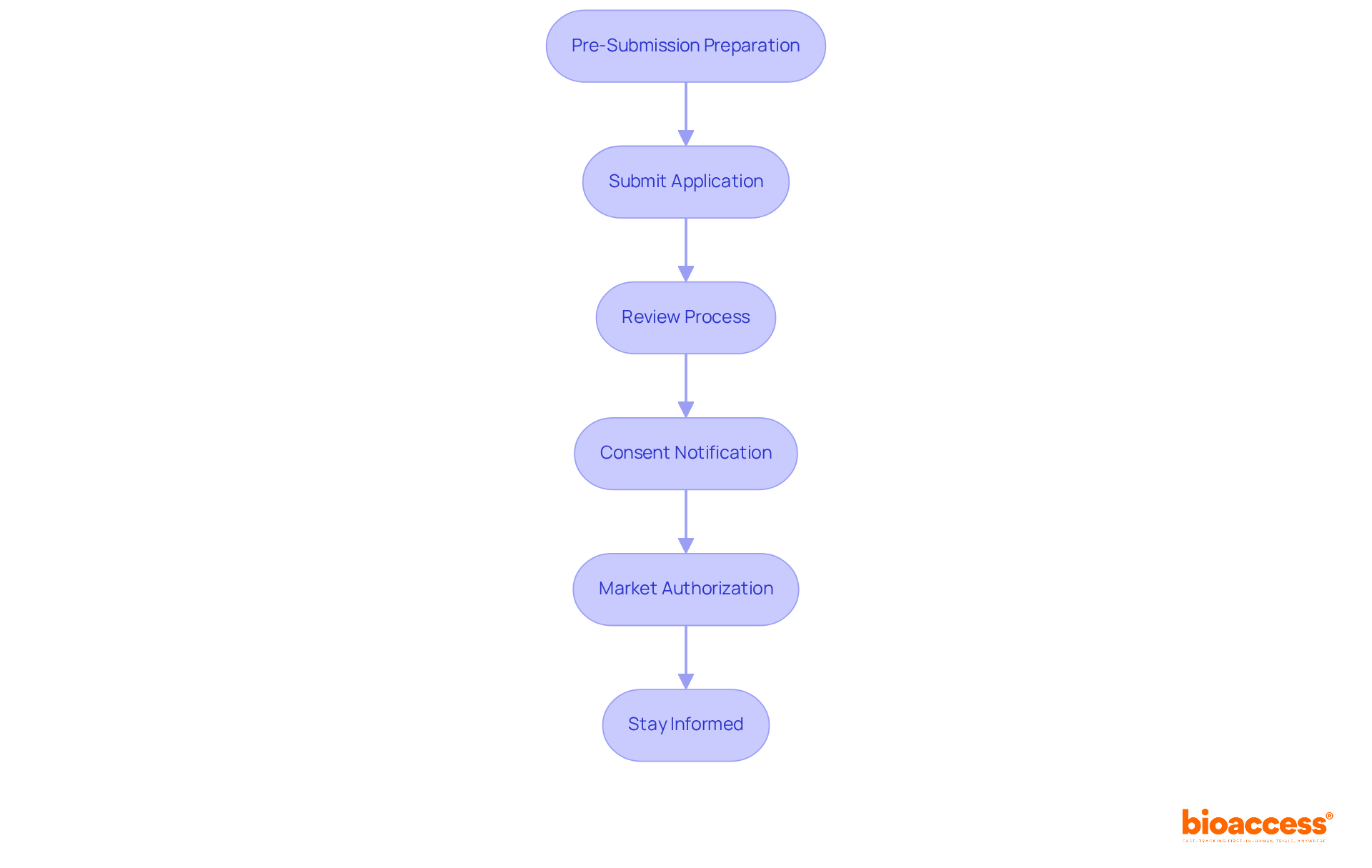
Ensuring compliance and safety monitoring is crucial in the biologic drug approval procedure in Macedonia. Here are essential strategies to implement:
Establish a Compliance Team: Create a dedicated group responsible for adhering to regulatory requirements throughout the approval procedure. This team should comprise experts in regulatory affairs, clinical research, and quality assurance, ensuring a comprehensive understanding of the landscape.
Regular Audits and Reviews: Conduct frequent audits of processes and documentation to align with MALMED guidelines and EU regulations. This proactive approach helps identify potential regulatory issues early, safeguarding the integrity of the trial.
Safety Monitoring Protocols: Implement rigorous safety monitoring protocols during clinical trials, including systematic adverse event reporting and data collection. Such measures are crucial for ensuring patient safety and upholding ethical standards. For instance, case studies have shown that robust safety monitoring can significantly reduce risks associated with adverse events, enhancing participant welfare and trial credibility.
Training and Education: Provide continuous training for your team on regulatory requirements and best practices in compliance. Keeping your team informed fosters high standards throughout the approval process, which is vital for maintaining participant safety and data integrity.
Engage with Regulatory Authorities: Maintain open lines of communication with MALMED and other relevant authorities. This engagement promotes smoother interactions and clarifies adherence expectations, which is especially important as Macedonia aligns its protocols, including the biologic drug approval procedure in Macedonia, with evolving EU standards.
By prioritizing compliance and safety monitoring, you enhance the credibility of your application and contribute to the overall success of the biologic drug approval procedure in Macedonia.
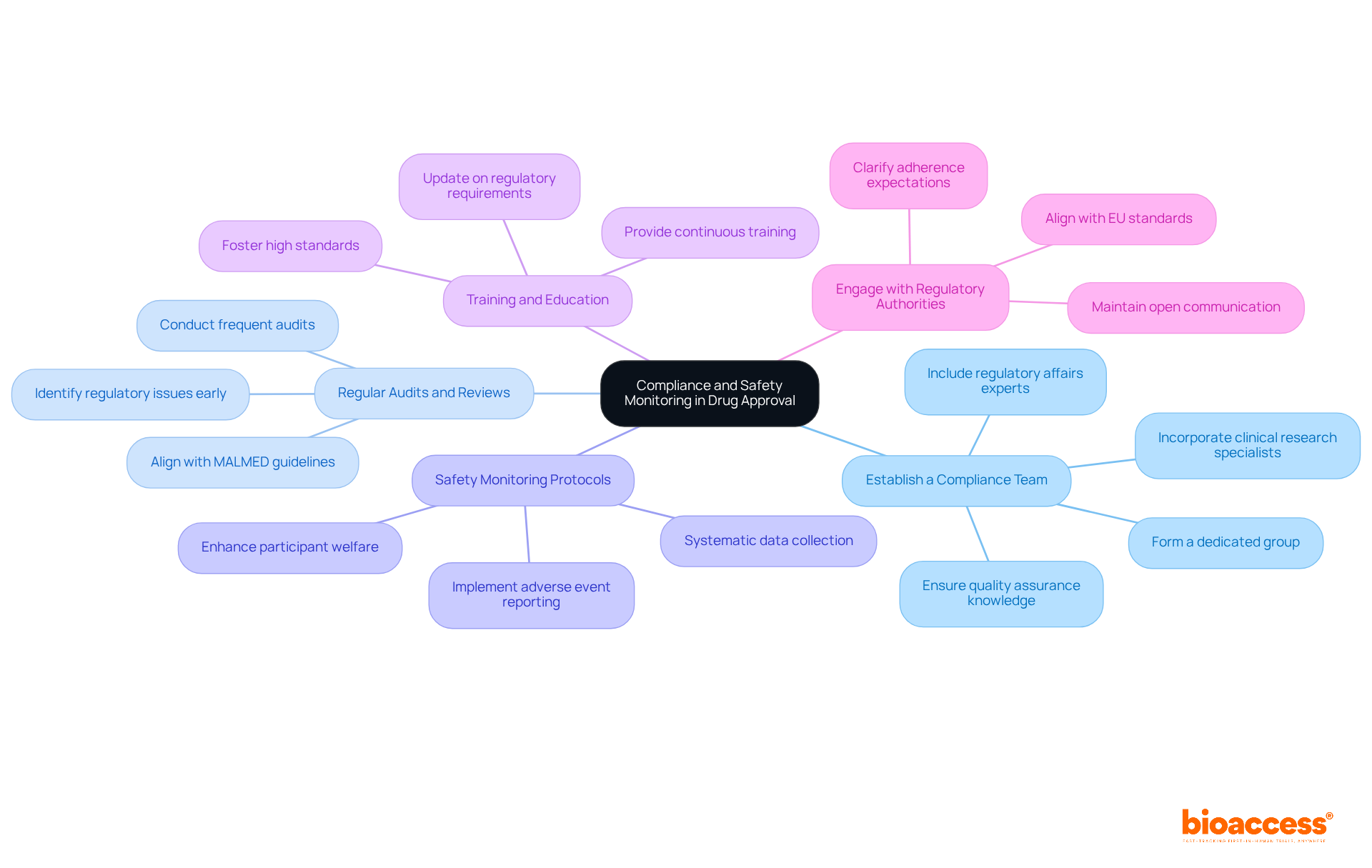
To expedite the biologic drug approval process in Macedonia, consider these effective strategies:
Utilize Fast-Track Designations: Investigate whether your product qualifies for any fast-track designations or expedited pathways offered by the Macedonian Agency for Medicines and Medical Devices (MALMED). These designations can significantly reduce review times, improving your chances of prompt acceptance.
Streamline Documentation: Ensure that all documentation is complete, accurate, and well-organized prior to submission. This meticulous preparation minimizes the risk of delays caused by requests for additional information, which can prolong the approval timeline.
Engage in Pre-Submission Meetings: Schedule pre-submission meetings with MALMED to discuss your application and gather feedback. These discussions can clarify expectations and assist in identifying potential issues early on, facilitating smoother submissions.
Leverage Existing Data: If applicable, utilize existing clinical data from previous studies or similar products to bolster your application. This approach can strengthen your case and potentially shorten the review timeline by demonstrating prior efficacy and safety.
Plan for Post-Approval Requirements: Anticipate any post-approval commitments and prepare to address them proactively. Being prepared for these requirements can prevent delays in market entry and ensure a smoother transition from validation to commercialization.
By implementing these strategies, you can enhance the efficiency of the biologic drug approval procedure in Macedonia and expedite the market entry of your biologic drug.
Navigating the biologic drug approval procedure in Macedonia is not just a task; it’s a critical endeavor that demands a thorough understanding of the regulatory landscape and a strategic approach to streamline the process. By familiarizing oneself with key regulations, particularly those from the Macedonian Agency for Medicines and Medical Equipment, stakeholders can ensure compliance and significantly enhance the likelihood of successful submissions.
This article outlines essential steps for achieving a smooth approval process. From pre-submission preparation to maintaining open communication with regulatory authorities, and implementing robust safety monitoring protocols, each step is vital. Emphasizing compliance is crucial; the strategies presented here not only enhance the credibility of applications but also facilitate timely market entry for biologics.
Ultimately, successfully navigating the biologic drug approval procedure in Macedonia hinges on more than just understanding the regulatory framework. It requires proactive engagement and strategic planning. By adopting best practices and staying informed about evolving guidelines, stakeholders can dramatically improve approval timelines and contribute to the advancement of biologic therapies in the region.
What is the primary organization overseeing the biologic drug approval procedure in Macedonia?
The primary organization supervising the biologic drug approval procedure in Macedonia is the Macedonian Agency for Medicines and Medical Equipment.
What law outlines the requirements for the registration and acceptance of medicinal products, including biologics?
The Law on Medicines and Medical Devices outlines the requirements for the registration and acceptance of medicinal products, including biologics.
What key aspects should be understood regarding the Law on Medicines and Medical Devices?
It is crucial to understand the stipulations regarding safety, efficacy, and quality as outlined in the Law on Medicines and Medical Devices.
Where can specific guidelines for submission requirements and documentation be found?
Specific guidelines detailing submission requirements, necessary documentation, and the assessment procedure can be found in the guidelines provided by MALMED.
How does Macedonia's regulation of biologics relate to European Union standards?
Macedonia aligns its regulations with EU standards, so understanding EU directives related to biologics provides valuable insights into evaluation and expectations.
What should be known about the regulations concerning biosimilars?
If a biologic is a biosimilar, it is important to familiarize oneself with the abbreviated registration procedure and the data requirements to demonstrate comparability with the reference product.
Why is it important to interact with regulatory authorities during the biologic drug approval process?
Maintaining an open dialogue with MALMED is vital for addressing any concerns that may arise during the validation stage.
What is a useful tool to ensure all necessary documents are included in the submission?
Utilizing a submission checklist is a useful tool to ensure that all necessary documents are included, helping to prevent delays in the endorsement stage.
What is required for documents not in Macedonian?
All documents not in Macedonian must be accurately translated and certified to meet local requirements.