


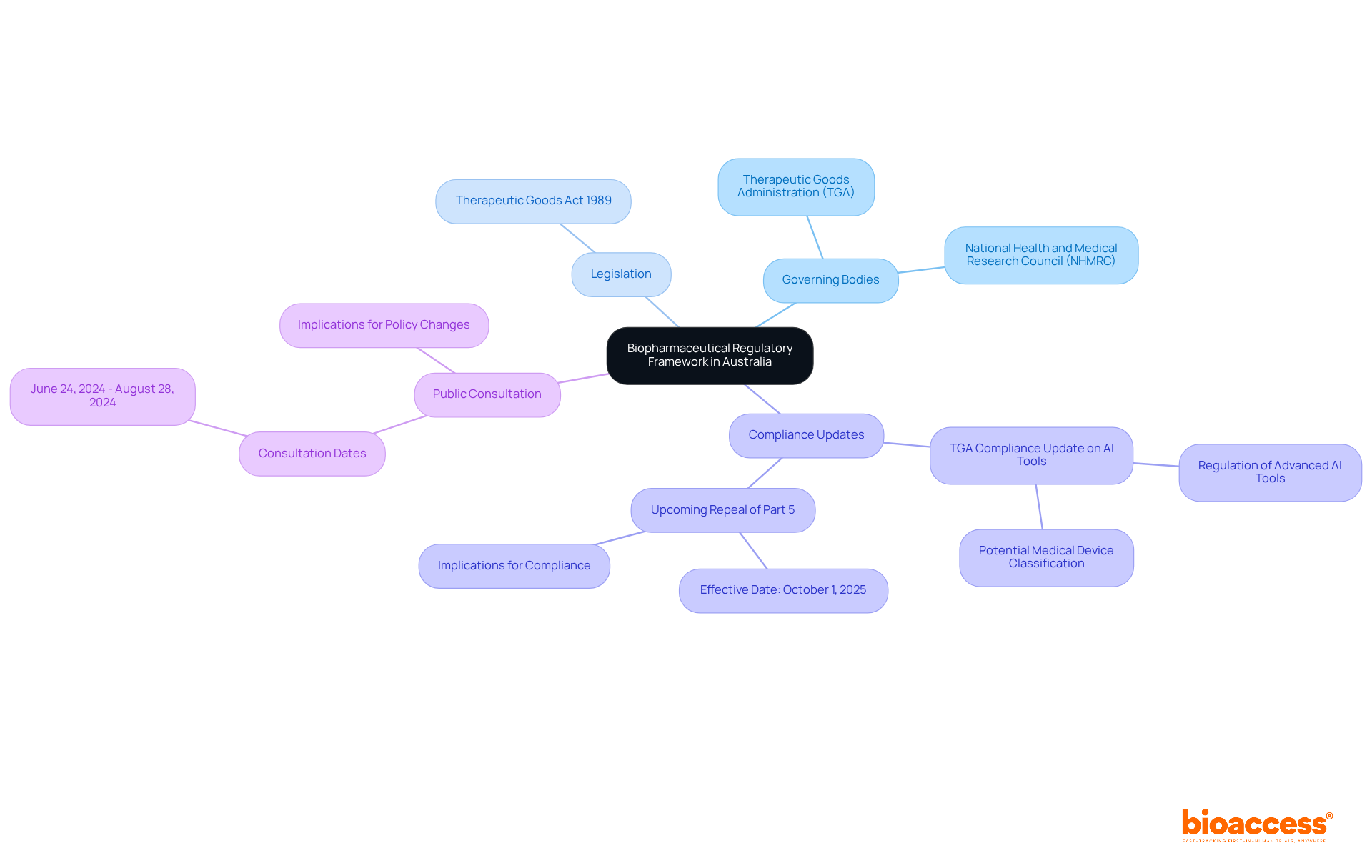
Navigating the complex landscape of biopharmaceutical regulations in Australia is essential for professionals in clinical research. This guide outlines the key components of the regulatory framework, providing insights into the requirements established by governing bodies such as the Therapeutic Goods Administration (TGA) and the National Health and Medical Research Council (NHMRC). With the rapid evolution of regulations and significant updates anticipated in 2025, organizations must consider:
Navigating the biopharmaceutical regulatory roadmap in Australia is crucial for anyone involved in clinical research. Understanding the key governing bodies, such as the Therapeutic Goods Administration (TGA) and the National Health and Medical Research Council (NHMRC), is essential. Familiarize yourself with the Therapeutic Goods Act 1989, which governs the approval and regulation of therapeutic goods. Notably, on September 10, 2025, the TGA released a compliance update emphasizing the regulation of advanced AI tools as potential medical devices under this Act.
Additionally, reviewing the biopharmaceutical regulatory roadmap in Australia will provide insights into the specific requirements for biological products. This foundational knowledge will not only help you recognize the necessary actions for compliance but also simplify your official filings. Furthermore, the upcoming repeal and replacement of Part 5 on October 1, 2025, underscores the importance of staying informed about evolving regulations.
Participating in the public consultation from June 24, 2024, to August 28, 2024, can offer valuable insights into policy changes and their implications for your organization. By engaging with these developments, you position yourself to effectively navigate the complexities of the biopharmaceutical regulatory roadmap in Australia.
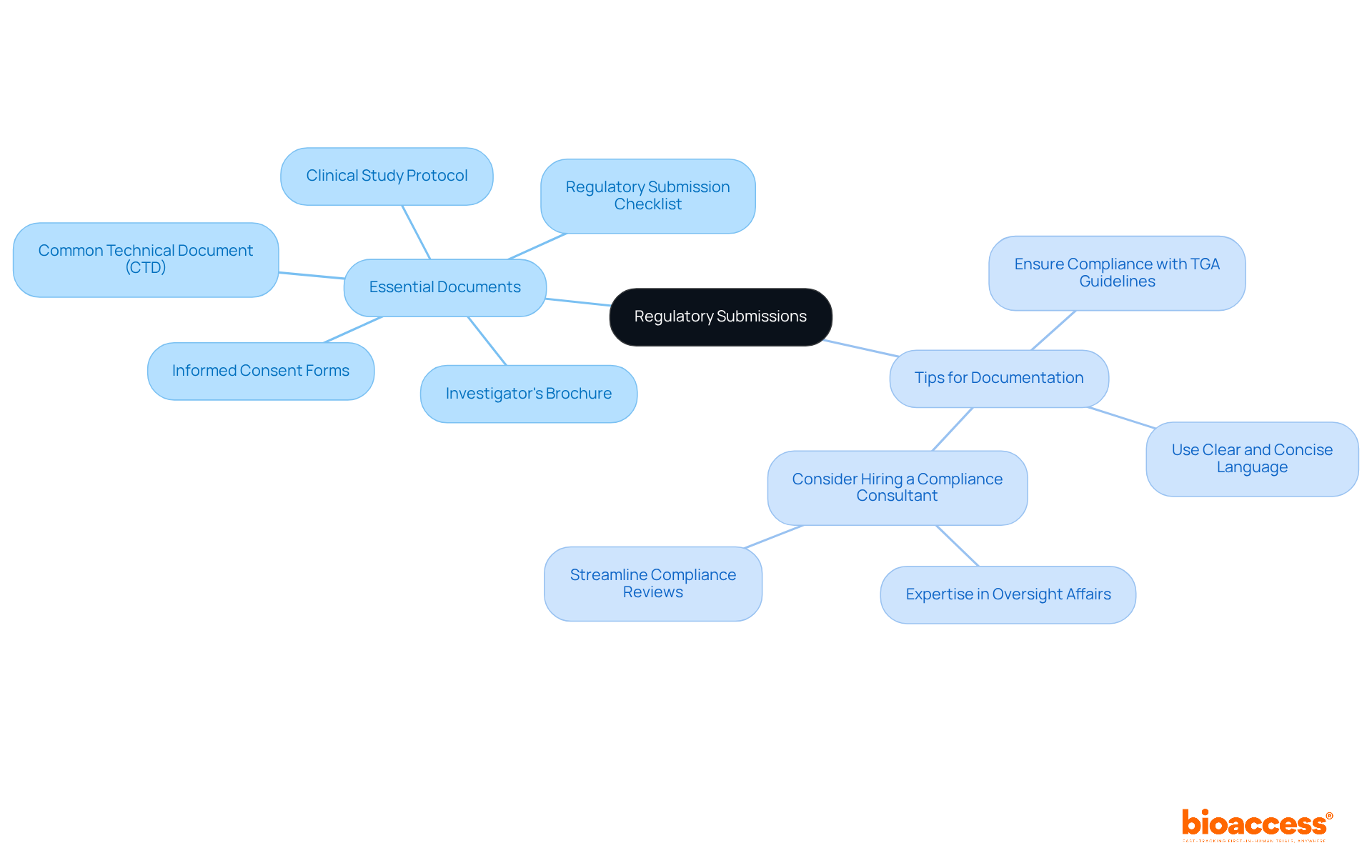
When preparing for regulatory submissions in Australia, it’s crucial to compile the following essential documents:
Tips for Documentation:
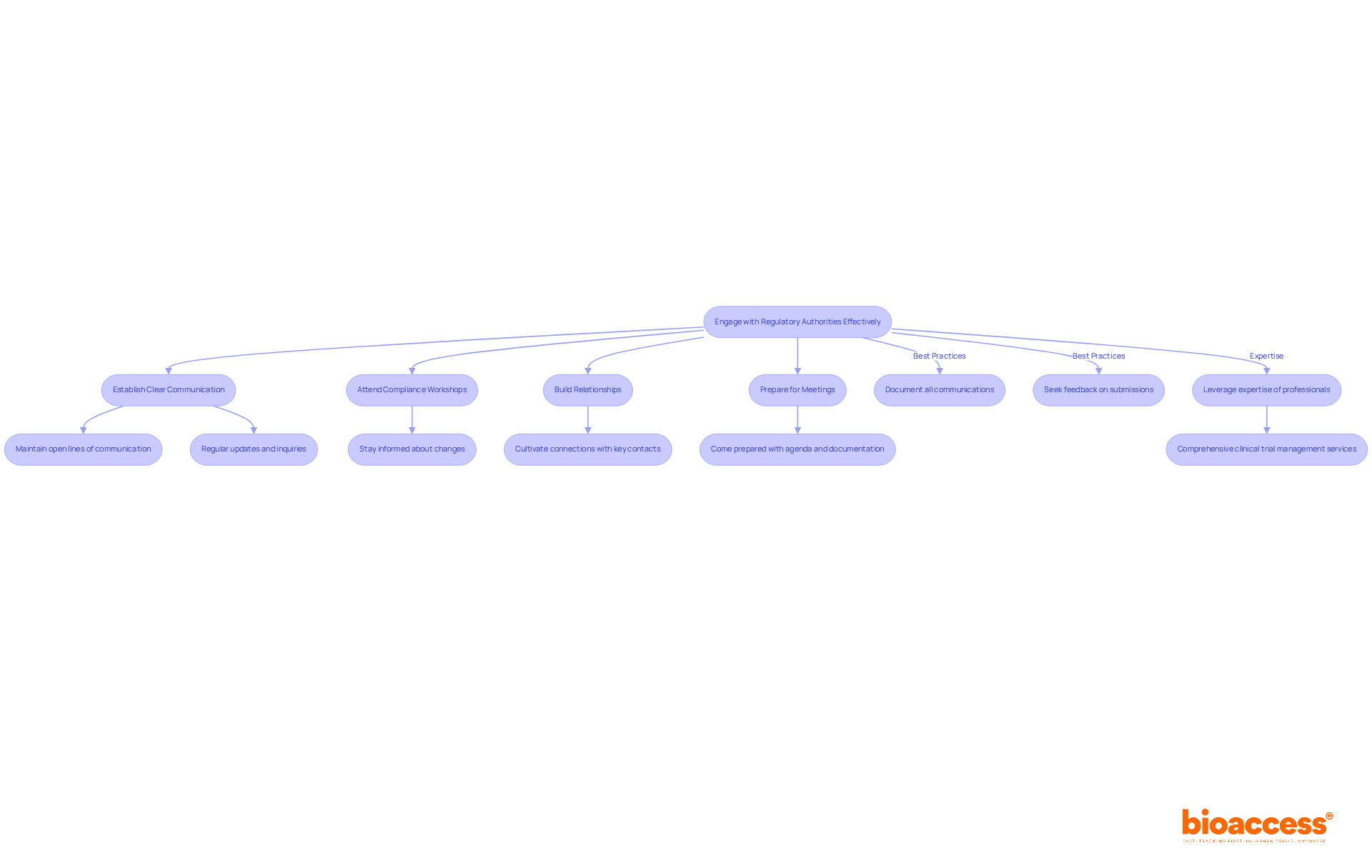
To engage effectively with regulatory authorities in Australia, consider these strategies:
Establish Clear Communication: Maintain open lines of communication with the TGA and other relevant bodies. Regular updates and inquiries can clarify expectations and requirements.
Attend Compliance Workshops: Participate in workshops and seminars hosted by oversight agencies to stay informed about changes and best practices.
Build Relationships: Cultivate connections with key contacts within governing bodies. This can enable smoother interactions and offer insights into the compliance process.
Prepare for Meetings: When meeting with regulators, come prepared with a clear agenda, relevant documentation, and specific questions to maximize the effectiveness of the discussion.
Best Practices:
Furthermore, leveraging the expertise of professionals like Ana Criado, who possesses considerable experience in compliance matters and clinical study oversight, can provide valuable perspectives on navigating the complexities of the biopharmaceutical regulatory roadmap in Australia. Comprehensive clinical trial management services-including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting-are essential for ensuring successful interactions with oversight bodies.
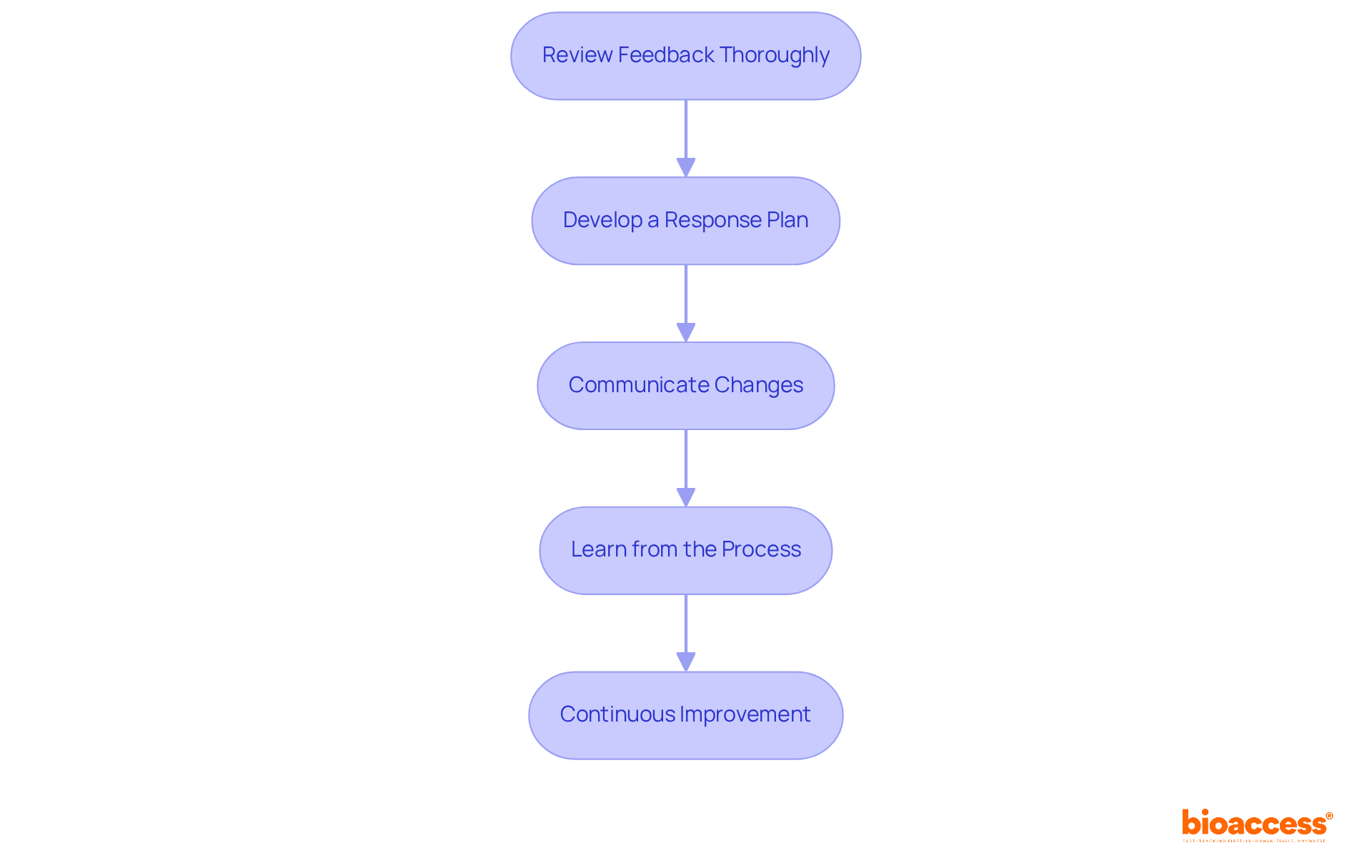
After submitting your regulatory documents, it’s crucial to closely monitor any feedback from regulatory authorities. Here’s how to effectively adapt to their feedback:
Continuous Improvement:
At Bioaccess, we recognize the complexities of the biopharmaceutical regulatory roadmap in Australia and offer comprehensive clinical trial management services. Our offerings include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. With our expertise, you can navigate these challenges effectively, fast-tracking your innovations in medtech, biopharma, and radiopharma.
Navigating the biopharmaceutical regulatory landscape in Australia demands a thorough grasp of its frameworks, key governing bodies, and the ever-evolving regulations. Understanding the roles of the Therapeutic Goods Administration (TGA) and the National Health and Medical Research Council (NHMRC) is paramount, as these entities are vital in ensuring compliance and facilitating the approval of therapeutic goods. With significant regulatory shifts anticipated in 2025, stakeholders must remain proactive and engaged with these developments.
This article underscores several critical aspects of this navigation process, including the preparation of essential documentation such as:
Effective engagement with regulatory authorities through clear communication, participation in workshops, and relationship-building is highlighted as a best practice for smoother interactions. Additionally, the importance of monitoring and adapting to feedback from regulators is crucial for continuous improvement and compliance.
Ultimately, the journey through the biopharmaceutical regulatory roadmap in Australia transcends mere compliance; it is about fostering innovation and ensuring patient safety. By leveraging expert insights and committing to compliance, organizations can adeptly navigate these complexities. Embracing the evolving landscape and actively participating in consultations and workshops will not only enhance individual submissions but also contribute to the overall advancement of biopharmaceuticals in Australia.
What is the significance of understanding the biopharmaceutical regulatory framework in Australia?
Understanding the biopharmaceutical regulatory framework is crucial for anyone involved in clinical research, as it helps in navigating the regulatory roadmap and ensuring compliance with necessary regulations.
Which key governing bodies oversee biopharmaceutical regulations in Australia?
The key governing bodies include the Therapeutic Goods Administration (TGA) and the National Health and Medical Research Council (NHMRC).
What legislation governs the approval and regulation of therapeutic goods in Australia?
The Therapeutic Goods Act 1989 governs the approval and regulation of therapeutic goods in Australia.
What recent update did the TGA release regarding advanced AI tools?
On September 10, 2025, the TGA released a compliance update emphasizing the regulation of advanced AI tools as potential medical devices under the Therapeutic Goods Act 1989.
Why is it important to review the biopharmaceutical regulatory roadmap in Australia?
Reviewing the roadmap provides insights into the specific requirements for biological products, helps recognize necessary actions for compliance, and simplifies official filings.
What upcoming regulatory change should stakeholders be aware of?
The repeal and replacement of Part 5 on October 1, 2025, is an important regulatory change that stakeholders should be informed about.
How can participating in public consultations benefit organizations involved in biopharmaceuticals?
Participating in public consultations, such as the one from June 24, 2024, to August 28, 2024, can provide valuable insights into policy changes and their implications for organizations, aiding in effective navigation of the regulatory landscape.