


Navigating ANVISA regulations for clinical trials requires a comprehensive understanding of the agency's role, key regulatory requirements, and common challenges faced in Brazil. This article highlights the critical importance of thorough preparation, which includes:
Such measures can significantly streamline the approval process and enhance the likelihood of successful research implementation.
In the intricate world of clinical trials, understanding the regulatory landscape is paramount for success, particularly in Brazil, where ANVISA, the Brazilian Health Regulatory Agency, serves as the guardian of public health. With its comprehensive oversight, ANVISA ensures that clinical trials adhere to both national and international standards, safeguarding the rights and safety of participants while facilitating access to innovative therapies. As researchers embark on the journey of conducting clinical trials, familiarity with ANVISA's processes and requirements is not merely beneficial—it's essential.
This article delves into the critical role ANVISA plays, the key regulatory requirements researchers must navigate, and the strategies to overcome common challenges in securing approval for clinical studies. By equipping themselves with this knowledge, researchers can enhance their likelihood of success and contribute meaningfully to the advancement of medical science in Brazil.
ANVISA, the Brazilian Health Regulatory Agency, plays a pivotal role in supervising research studies in Brazil, ensuring adherence to both national and international standards to safeguard public health. Its primary responsibilities encompass:
Understanding these functions is essential for researchers navigating ANVISA regulations for clinical trials effectively. Familiarity with the agency's procedures and navigating ANVISA regulations for clinical trials can significantly expedite the approval timeline, thereby increasing the likelihood of successful study implementation, as the impact of the agency's regulations on research success rates is substantial. By allowing simultaneous evaluations of research study applications by regulatory authorities and ethics committees, the agency has streamlined the approval process, reducing delays in initiating research. This approach facilitates prompt access to new treatments and therapies for patients, underscoring the regulatory agency's critical role in medical research.
Recent updates from the agency underscore its commitment to transparency and efficiency, particularly regarding health inspections and Good Manufacturing Practice (GMP) certificates. Detailed knowledge of AREE requirements for health inspections and GMP certificates is vital for ensuring compliance and operational excellence in research studies. Furthermore, reports indicate that ethics committees (CEPs) must retain all pertinent records for three years following the conclusion of a study, ensuring accountability and thorough documentation. It is also crucial for researchers to prepare reports on biobanking for research purposes in accordance with specified guidelines, as navigating ANVISA regulations for clinical trials is an integral component of the compliance framework.
In conclusion, a thorough understanding of the agency's role and the current compliance landscape as of 2025 is imperative for researchers. By adhering to bioaccess®'s guidelines and leveraging the comprehensive management services for studies—including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—research initiatives can achieve greater success and significantly advance medical technology in Brazil. Katherine Ruiz's expertise in regulatory affairs for medical devices and in vitro diagnostics further bolsters the support available to navigate these intricate requirements.
Navigating ANVISA regulations for clinical trials requires a comprehensive understanding of several key requirements that are crucial for research success in Brazil, including the submission of Clinical Trial Applications (CTA), which must include a detailed study protocol, objectives, and methodology. This step is essential for initiating the regulatory process.
With 70% of the population residing two hours or more from an academic medical center, decentralized studies can significantly improve participant recruitment and diversity, addressing logistical challenges in clinical research. By integrating decentralized methods, researchers can enhance efficiency in studies while navigating ANVISA regulations for clinical trials to fulfill the necessary requirements. Furthermore, addressing screen failures—averaging around $1,200 per failure—through improved pre-screening and targeted recruitment strategies can notably reduce costs and enhance study efficiency. Understanding these regulatory requirements is vital for navigating ANVISA regulations for clinical trials, ensuring compliance, and facilitating a smoother approval process. At bioaccess®, we leverage over 20 years of expertise in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies. Our commitment ensures that clients navigate the complexities of clinical trials in Latin America effectively, with a customized approach tailored to their specific needs.
Preparing and submitting a Clinical Trial Application (CTA) to ANVISA requires careful attention to several essential steps:
The likelihood of a successful application can be significantly improved by navigating ANVISA regulations for clinical trials and adhering to these steps. Significantly, the success rates of trial applications submitted to the agency have been positively impacted by navigating ANVISA regulations for clinical trials, which feature clearer regulatory definitions and a dedication to transparency. As stated by ANVISA, "With these changes, Brazil is positioning itself as an important hub for the development of new therapies, benefiting patients and boosting science in the country." These changes position Brazil as an essential center for medical research, enhancing patient access to innovative therapies while promoting scientific progress. Moreover, sponsors should keep research data for a minimum of two years if the investigational product’s development is halted. Employing the Clinical Trials tool provides public access to the status of each study, including location and responsible researchers, highlighting the significance of remaining informed throughout the process.
For those navigating ANVISA regulations for clinical trials, bioaccess® offers extensive management services for research studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. Collaborating with bioaccess can offer the knowledge and tailored strategy required to effectively oversee your clinical trial and guarantee adherence to regulatory standards.
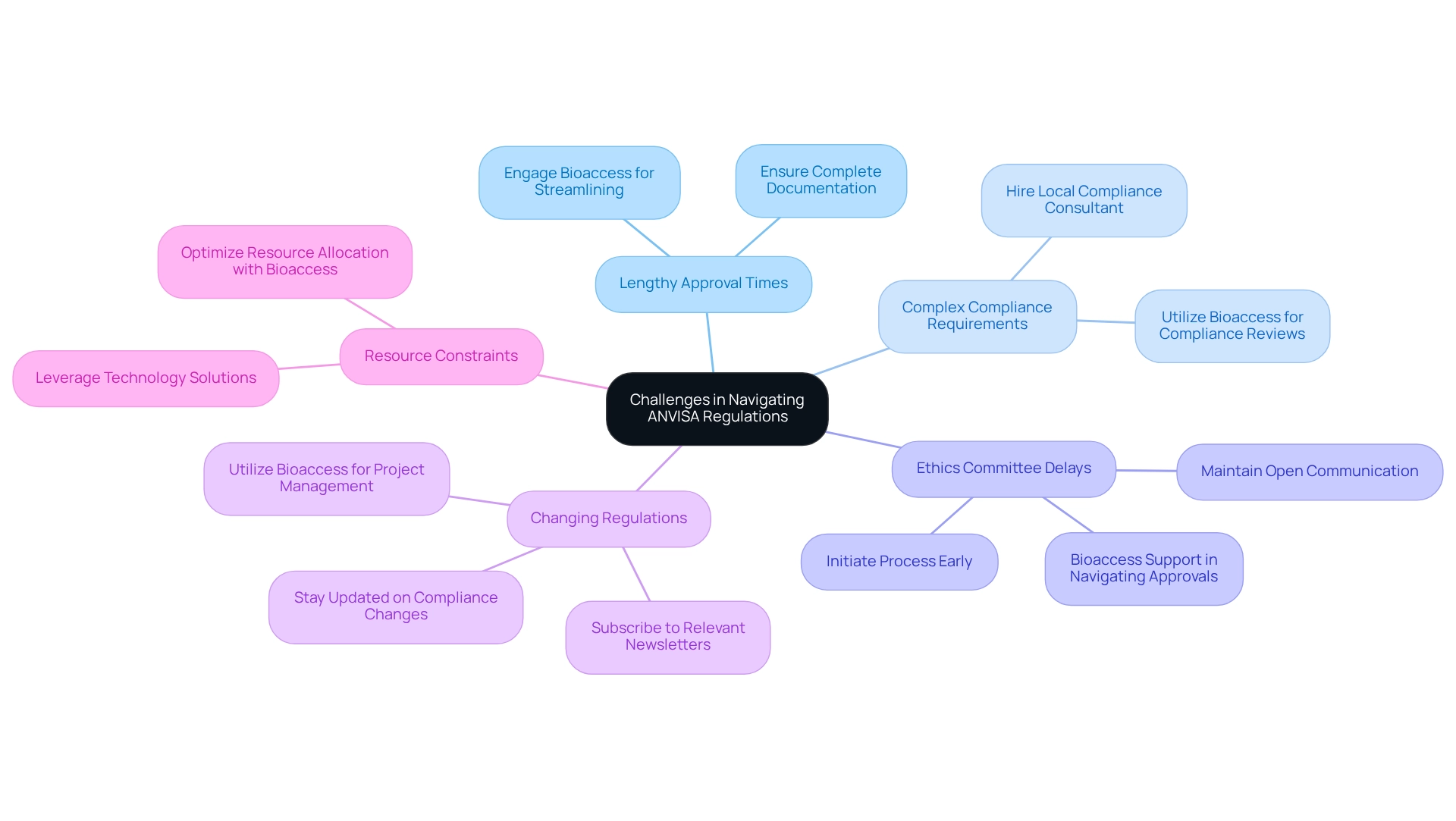
Navigating ANVISA regulations for clinical trials presents several challenges that are crucial for the success of clinical research. Common issues include:
Addressing these challenges effectively is essential for researchers aiming to achieve their objectives.
Lengthy approval times can significantly hinder progress. ANVISA's review process is often prolonged, making it vital to ensure that all documentation is complete and accurate prior to submission. This proactive approach can mitigate delays caused by requests for additional information. Engaging Bioaccess can streamline this process, leveraging their expertise in trial setup, including feasibility studies and project management.
Complex compliance requirements further complicate the landscape. The oversight framework can be intricate, necessitating the hiring of a local compliance consultant. Bioaccess offers valuable insights and tailored guidance specific to your study, including compliance reviews, feedback on study documents, and assistance with import permits. Additionally, delays in obtaining ethics committee approval can impact timelines. Initiating this process early and maintaining open communication with the committee can expedite approvals. Bioaccess provides support in trial setup, assisting in navigating these approvals efficiently.
Compliance with changing regulations is another critical concern. As regulations evolve, they can impact ongoing studies. Staying updated on compliance changes through official communications is essential; subscribing to relevant newsletters can be beneficial. Utilizing Bioaccess's services ensures adherence to the latest regulations through their comprehensive project management.
Resource constraints can also hinder compliance efforts. Researchers should consider leveraging technology solutions for document management and regulatory tracking to streamline processes. Bioaccess offers robust project management services that optimize resource allocation and enhance compliance efforts. By proactively addressing these challenges and leveraging the comprehensive clinical trial management services offered by Bioaccess, including feasibility studies and import permits, researchers can significantly enhance their chances of navigating ANVISA regulations for clinical trials successfully.
The regulatory landscape surrounding clinical trials in Brazil is intricate, with ANVISA at its helm, ensuring that trials comply with both national and international standards. Understanding ANVISA's critical role—ranging from evaluating clinical trial applications to enforcing ethical standards—is essential for researchers aiming to enhance their success rates. By familiarizing themselves with ANVISA's processes and leveraging resources such as bioaccess®, researchers can significantly streamline the approval timeline and contribute to the advancement of medical science.
Navigating key regulatory requirements, such as obtaining ethics committee approval and adhering to Good Clinical Practices (GCP), is crucial for the successful execution of clinical trials. A proactive approach to preparing and submitting Clinical Trial Applications (CTAs) can mitigate common challenges, including lengthy approval times and complex regulatory nuances. By ensuring comprehensive documentation and maintaining open communication with ethics committees, researchers can enhance their likelihood of obtaining timely approvals.
Ultimately, overcoming the challenges posed by ANVISA regulations requires diligence, strategic planning, and the support of experienced partners like bioaccess®. By embracing these strategies and remaining informed about the regulatory environment, researchers can not only facilitate smoother clinical trial processes but also pave the way for innovative therapies that benefit patients across Brazil. The commitment to regulatory compliance and ethical standards will be paramount in driving forward the future of clinical research in the country.
What is ANVISA and what role does it play in research studies in Brazil?
ANVISA, the Brazilian Health Regulatory Agency, supervises research studies in Brazil, ensuring adherence to national and international standards to safeguard public health.
What are the primary responsibilities of ANVISA?
ANVISA's primary responsibilities include assessing research applications, overseeing ongoing studies, and ensuring compliance with ethical standards.
How can familiarity with ANVISA regulations benefit researchers?
Familiarity with ANVISA regulations can expedite the approval timeline for clinical trials, increasing the likelihood of successful study implementation.
What recent updates have been made by ANVISA regarding health inspections and GMP certificates?
Recent updates from ANVISA emphasize transparency and efficiency in health inspections and Good Manufacturing Practice (GMP) certificates.
How long must ethics committees (CEPs) retain records following the conclusion of a study?
Ethics committees (CEPs) must retain all pertinent records for three years after the conclusion of a study to ensure accountability and thorough documentation.
What is the significance of preparing reports on biobanking for research purposes?
Preparing reports on biobanking in accordance with specified guidelines is crucial for navigating ANVISA regulations and ensuring compliance in research studies.
What are some services offered by bioaccess® to assist researchers?
Bioaccess® offers comprehensive management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting.
Why is understanding ANVISA's role and the compliance landscape important for researchers?
A thorough understanding of ANVISA's role and current compliance landscape is imperative for researchers to navigate regulations effectively and achieve greater success in their research initiatives.