


The article delineates the pivotal role of the Therapeutic Goods Administration (TGA) in Australia, tasked with ensuring the safety, efficacy, and quality of therapeutic goods, including medicines and medical devices. It elucidates the TGA's meticulous evaluation processes and ongoing monitoring, underscoring its adaptability to emerging health needs, particularly in the context of the COVID-19 pandemic. This exemplifies the TGA's essential function in safeguarding public health while facilitating timely access to innovative treatments. The TGA's comprehensive approach not only protects citizens but also fosters trust in the healthcare system, reinforcing its significance in the landscape of clinical research.
The Therapeutic Goods Administration (TGA) serves as a crucial guardian of public health in Australia, overseeing the safety, efficacy, and quality of therapeutic goods, which encompass medicines and medical devices. As the healthcare landscape evolves—particularly with the emergence of Medtech and Biopharma innovations—grasping the TGA's regulatory framework is essential for stakeholders seeking to navigate this intricate environment. However, given the TGA's stringent approval processes and post-market monitoring, how can companies effectively align their strategies to ensure timely access to their products while upholding compliance?
The Therapeutic Goods Administration Australia serves as the pivotal oversight body in Australia, ensuring the safety, efficacy, and quality of therapeutic goods, including medicines, medical devices, and biological substances. Established under the Therapeutic Goods Act 1989, the Therapeutic Goods Administration Australia plays a crucial role in evaluating items prior to market entry and ensuring compliance through comprehensive post-market monitoring. This encompasses the assessment of clinical trials, the approval of new medicines, and the regulation of advertising practices, all aimed at protecting public health.
As of 2025, the TGA is adapting its oversight framework to align with the dynamic needs of the Medtech and Biopharma sectors. Recent updates underscore the importance of stakeholder involvement, particularly regarding the use of unapproved medicinal cannabis items, which could influence future policy developments. The TGA's commitment to transparency and safety is reflected in its regular updates on assessments and adverse event reporting, ensuring that both consumers and healthcare professionals remain well-informed.
Understanding the Therapeutic Goods Administration Australia’s regulatory landscape is crucial for stakeholders in the Medtech and Biopharma sectors, as it significantly impacts development timelines and market entry strategies. The Therapeutic Goods Administration Australia employs a risk-based approach to evaluate market authorization applications, allowing lower-risk items to undergo streamlined assessments while subjecting higher-risk items to more rigorous scrutiny. This classification system not only accelerates the approval process but also ensures that items are meticulously evaluated based on their associated risks.
The Therapeutic Goods Administration Australia influences not only initial approvals but also actively monitors therapeutic goods post-market, addressing any safety or quality issues that may emerge. This ongoing oversight is vital for maintaining public trust in health products and facilitating the timely delivery of innovative treatments to patients. As the therapeutic goods landscape continues to evolve, the Therapeutic Goods Administration Australia remains a cornerstone of regulatory integrity.
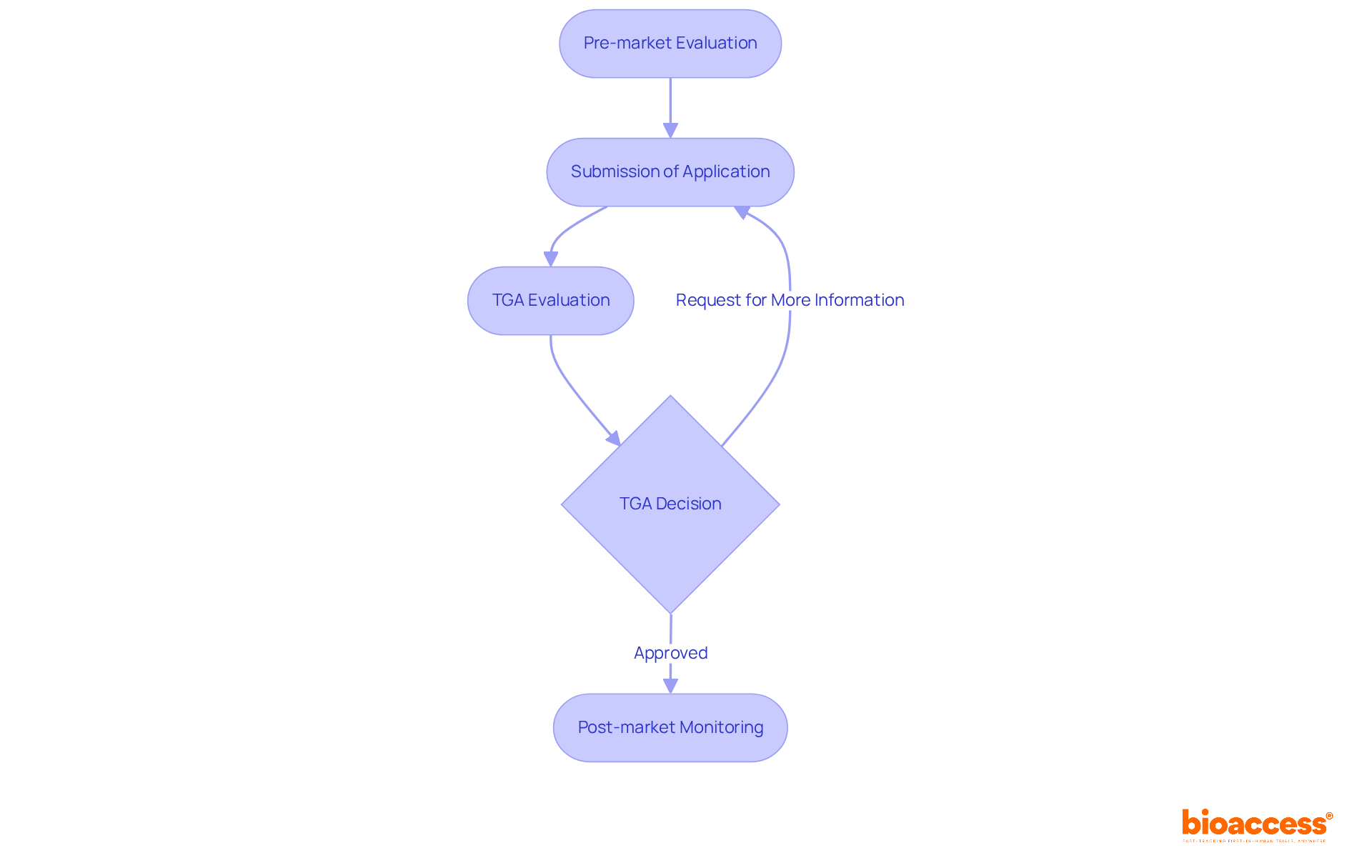
The approval process for therapeutic goods in Australia involves several critical stages:
Understanding these stages is crucial for achieving prompt and successful launches. Notably, the average processing times for various classes of medical devices illustrate the efficiency of the TGA, with Class 1 Sterile devices averaging just 4 days for ARTG inclusion. This efficiency underscores the importance of meticulous preparation and adherence to submission requirements, significantly enhancing the likelihood of successful applications.
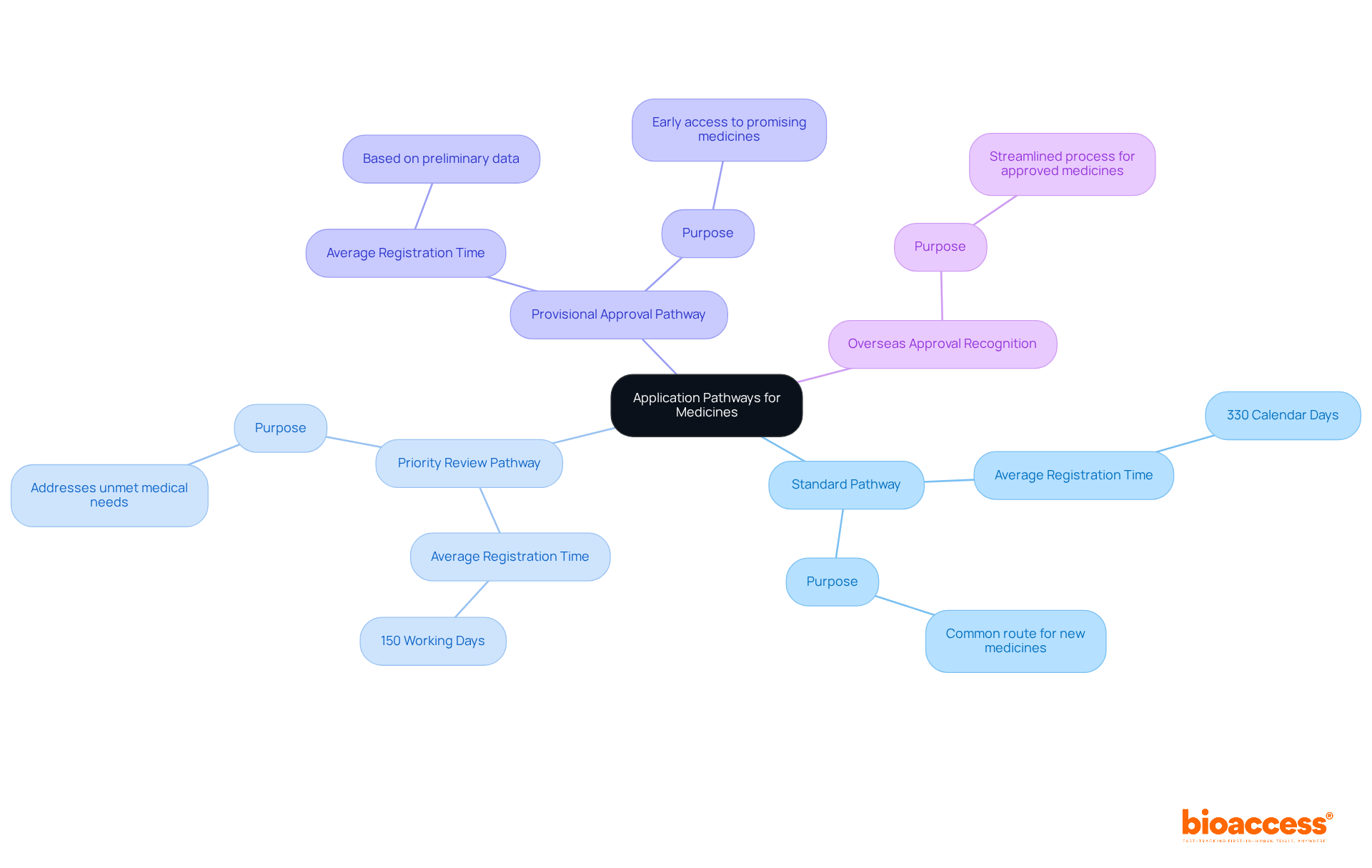
The TGA presents multiple application pathways for medicines, each specifically designed to meet distinct needs:
Standard Pathway: This is the most common route for new medicines, requiring comprehensive data on safety and efficacy. The average registration process for this pathway is structured to take approximately 330 calendar days, ensuring a meticulous evaluation.
Priority Review Pathway: Tailored for medicines addressing unmet medical needs, this pathway significantly expedites the review process, targeting a timeframe of 150 working days—up to three months less than the standard process. Recent enhancements to this pathway have streamlined evaluations further, facilitating quicker access to essential treatments.
Provisional Approval Pathway: This pathway enables early access to promising medicines based on preliminary clinical data, with the condition that further evidence must be submitted post-approval. This strategy is particularly advantageous for tackling urgent health challenges, such as during the COVID-19 pandemic, where provisional approvals expedited access to vaccines.
Overseas Approval Recognition: Medicines already sanctioned by comparable overseas regulators may benefit from a streamlined application process, alleviating the burden of additional data submission.
Understanding these pathways is crucial for companies aiming to align their compliance strategies with product development timelines in accordance with the Therapeutic Goods Administration Australia. High-quality applications are vital for ensuring that submissions are accepted for evaluation, as subpar or incomplete applications can result in delays or rejections. Moreover, leveraging comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting provided by Bioaccess, can enhance the processes involved in navigating the therapeutic goods administration Australia oversight landscape.
In response to the COVID-19 pandemic, the Therapeutic Goods Administration Australia implemented accelerated approval procedures for therapies and vaccines, significantly transforming the oversight environment. This initiative is crucial for clinical research, as it illustrates the adaptability of governance frameworks during urgent health crises. Key features of these fast-tracked pathways include:
A pertinent example of this expedited process is the provisional registration granted to Moderna's COVID-19 vaccine, showcasing the Therapeutic Goods Administration Australia’s commitment to facilitating timely access to critical treatments. Furthermore, bioaccess® offers a distinctive 6-8 week sprint method for approval, enabling quicker enrollment of treatment-naive cardiology or neurology groups, which can significantly enhance the efficiency of clinical trials. By overcoming compliance challenges and delivering comprehensive clinical trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—bioaccess® positions itself as a leader in navigating the complexities of clinical research in this evolving landscape.

Post-market monitoring and compliance are vital components of the TGA's regulatory framework, encompassing several key aspects that are crucial for ensuring the safety and efficacy of therapeutic goods.
Adverse Event Reporting: The TGA mandates that manufacturers report any negative incidents associated with their products, facilitating ongoing evaluations of risk. This requirement is essential for identifying potential hazard signals and ensuring that emerging risks are promptly addressed. For instance, the introduction of mandatory reporting for medical device adverse events in March 2023 has significantly improved the TGA's capability to monitor product integrity effectively. From March 2026, reporting by healthcare facilities will become mandatory, further strengthening this framework.
Compliance Inspections: The TGA conducts numerous compliance inspections annually across manufacturing facilities and clinical trial sites to ensure adherence to regulatory standards. In 2023, the TGA completed 202 focused and 26 in-depth signal investigations related to medicines, alongside 38 focused and 7 in-depth investigations related to vaccines. These inspections are crucial for confirming that companies uphold the quality and security of their therapeutic goods, as mandated by the therapeutic goods administration Australia throughout their lifecycle.
Market Surveillance: The TGA actively engages in market surveillance to ensure compliance with advertising and labeling regulations. This includes monitoring for misleading claims and taking necessary actions against non-compliance, thereby safeguarding public health and maintaining trust in therapeutic goods. The launch of the Australian Unique Device Identifier Database (AusUDID) aids in identifying specific device models during incidents, enhancing market surveillance efforts.
Risk Management: Employing robust risk management strategies, the TGA identifies and mitigates potential safety issues associated with therapeutic goods. This proactive approach ensures that products remain safe for public use, reflecting the TGA's commitment to protecting consumers. The TGA is also reviewing the definition of 'supply' to encompass digital access to technology, which is increasingly relevant in today's market.
In this context, bioaccess offers comprehensive clinical trial management services that include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. These services are designed to support companies in navigating the complexities of the TGA's regulatory framework, ensuring that they maintain their market authorization and uphold public health standards. Understanding these monitoring and compliance measures is essential for companies aiming to succeed in the Australian market.

The Therapeutic Goods Administration (TGA) functions as a pivotal regulatory authority in Australia, committed to ensuring the safety, quality, and efficacy of therapeutic goods. Its comprehensive role includes:
These functions are all essential for safeguarding public health. By adapting its oversight framework to address the evolving demands of the Medtech and Biopharma sectors, the TGA exemplifies a dedication to transparency and responsiveness in a rapidly changing landscape.
This article has provided key insights into the TGA's approval processes, detailing the various application pathways for medicines and the streamlined procedures introduced during the COVID-19 pandemic. Understanding these processes is crucial for stakeholders who seek to navigate the regulatory environment effectively. Moreover, the importance of post-market monitoring and compliance—particularly concerning:
underscores the TGA's ongoing responsibility in maintaining public trust.
Ultimately, the TGA's rigorous framework not only facilitates the timely delivery of innovative treatments but also reinforces the overarching goal of protecting public health. As the therapeutic goods landscape continues to evolve, stakeholders are encouraged to remain informed and engaged with the TGA's processes, ensuring alignment with the regulatory requirements that govern this critical sector. Embracing this knowledge will enhance compliance and contribute to the advancement of healthcare solutions in Australia.
What is the role of the Therapeutic Goods Administration (TGA) in Australia?
The TGA is responsible for ensuring the safety, efficacy, and quality of therapeutic goods, including medicines, medical devices, and biological substances. It evaluates items before market entry and conducts post-market monitoring to protect public health.
When was the Therapeutic Goods Administration established?
The TGA was established under the Therapeutic Goods Act 1989.
How does the TGA adapt to changes in the Medtech and Biopharma sectors?
As of 2025, the TGA is updating its oversight framework to meet the dynamic needs of these sectors, emphasizing stakeholder involvement, particularly regarding unapproved medicinal cannabis items.
What is the TGA's approach to market authorization applications?
The TGA employs a risk-based approach, allowing lower-risk items to undergo streamlined assessments while subjecting higher-risk items to more rigorous scrutiny.
What are the key stages in the approval process for therapeutic goods?
The approval process includes: 1. Pre-market Evaluation 2. Submission of Application 3. TGA Evaluation 4. TGA Decision 5. Post-market Monitoring
What does the submission of an application to the TGA involve?
Companies must provide a detailed application that includes clinical trial data, manufacturing information, and proposed labeling.
How does the TGA ensure thorough evaluation of applications?
The TGA conducts a comprehensive review of applications and often consults with external experts for a robust assessment.
What happens after the TGA evaluates an application?
The TGA either approves the item for market entry or requests additional information to address any concerns.
What does post-market monitoring by the TGA entail?
After approval, the TGA continues to oversee the item's integrity and efficacy through adverse event reporting and compliance checks.
How efficient is the TGA in processing applications for medical devices?
The average processing time for Class 1 Sterile devices is approximately 4 days for ARTG inclusion, highlighting the TGA's efficiency in the approval process.