


Navigating the landscape of clinical research in Bulgaria demands a solid understanding of the intricate ethics approval process, especially with new regulations set to take effect in 2025. This guide serves as a comprehensive roadmap for researchers seeking to secure multicenter ethics approvals. It details essential steps, from grasping the regulatory framework to gathering necessary documentation and engaging effectively with ethics committees.
However, the complexities of compliance and the potential for delays raise a critical question: how can researchers ensure their applications not only meet the required standards but also stand out in a competitive environment? By addressing these challenges head-on, this guide aims to empower researchers with the knowledge and strategies needed to navigate this evolving landscape.
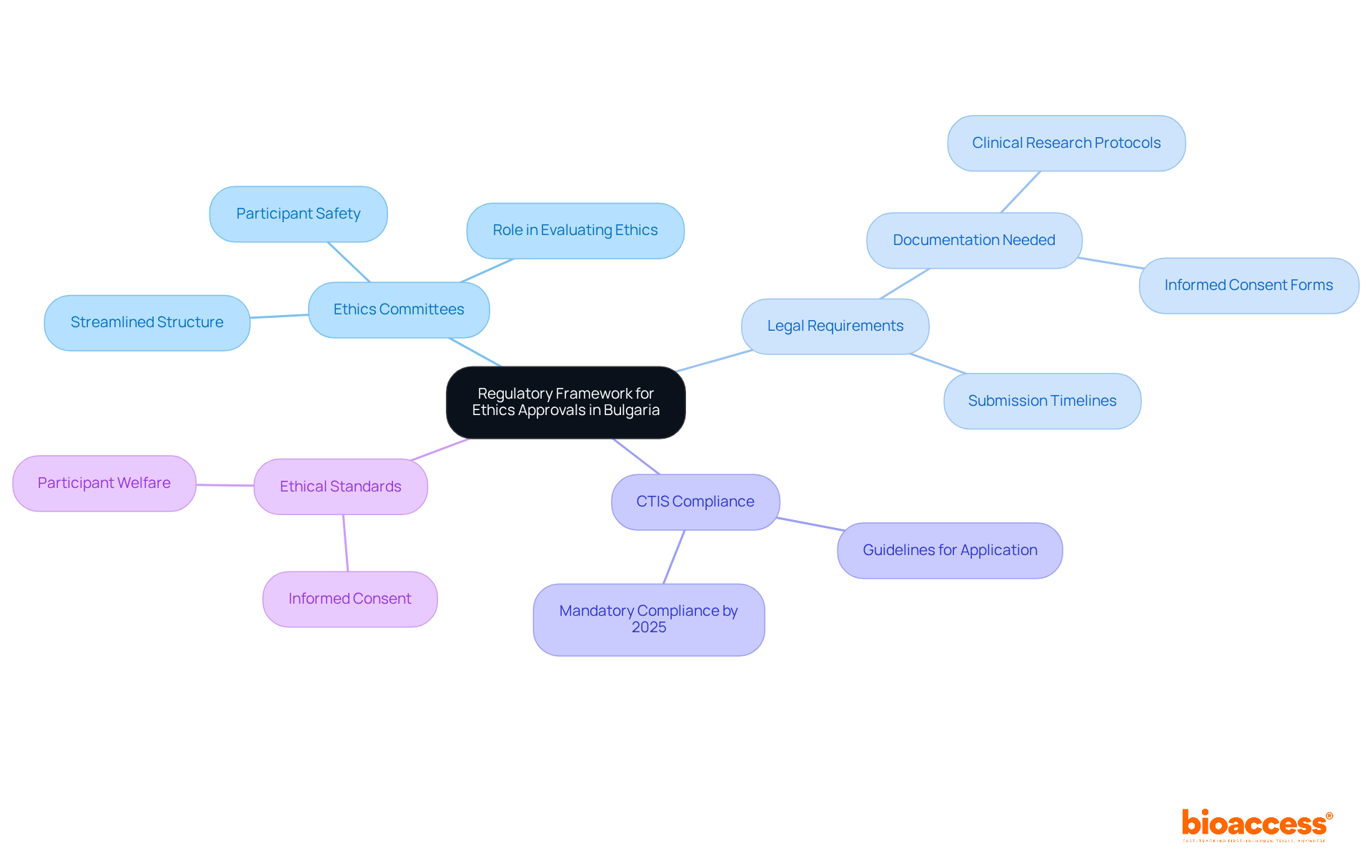
Obtaining multicenter ethics approvals in Bulgaria is crucial for successful clinical research, necessitating a comprehensive understanding of the regulatory framework governing clinical studies. Bulgaria adheres to the EU Clinical Studies Regulation (EU No 536/2014), which delineates the requirements for conducting clinical studies and securing ethical approvals. Key considerations include:
Ethics Committees: Familiarizing yourself with the structure and function of ethics committees in Bulgaria is essential. These groups play a pivotal role in evaluating the ethical aspects of clinical studies, ensuring participant safety and compliance with ethical standards. Notably, as of 2025, Bulgaria has streamlined its ethics committee structure, replacing the former three-tier system with a more efficient model that enhances oversight and accelerates the initiation of proceedings.
Legal Requirements: A thorough review of the legal requirements established by the Bulgarian Drug Agency (BDA) and the Ministry of Health is vital. This includes understanding the necessary documentation, such as clinical research protocols and informed consent forms, along with submission timelines. The BDA has recently updated its regulations to enhance the efficiency of the approval process, targeting a review duration of approximately 35 days.
CTIS Compliance: Starting January 31, 2025, all ongoing studies must comply with the Clinical Trials Information System (CTIS) regulations. Preparing your application according to these guidelines is critical to avoid unnecessary delays in the approval process. The CTIS aims to simplify submissions and improve overall study management across the EU.
Ethical Standards: Upholding ethical standards throughout the examination process is paramount, including ensuring informed consent and prioritizing participant safety. Understanding these moral responsibilities is essential for maintaining the integrity of your research and fostering a collaborative relationship with review boards.
By effectively navigating these elements, researchers can significantly enhance their chances of obtaining prompt approvals and successfully conducting clinical studies in Bulgaria.
Before submitting your ethics application, compiling all necessary documentation meticulously is crucial. The following documents are typically required:
Common documentation mistakes in clinical research submissions in Bulgaria can lead to delays in multicenter ethics approvals in Bulgaria. Issues such as incomplete forms, lack of necessary signatures, or failure to provide documents in the required languages can hinder the submission process. Therefore, attention to detail is paramount to ensure successful submissions of Clinical Trial Applications (CTAs) that include multicenter ethics approvals in Bulgaria.

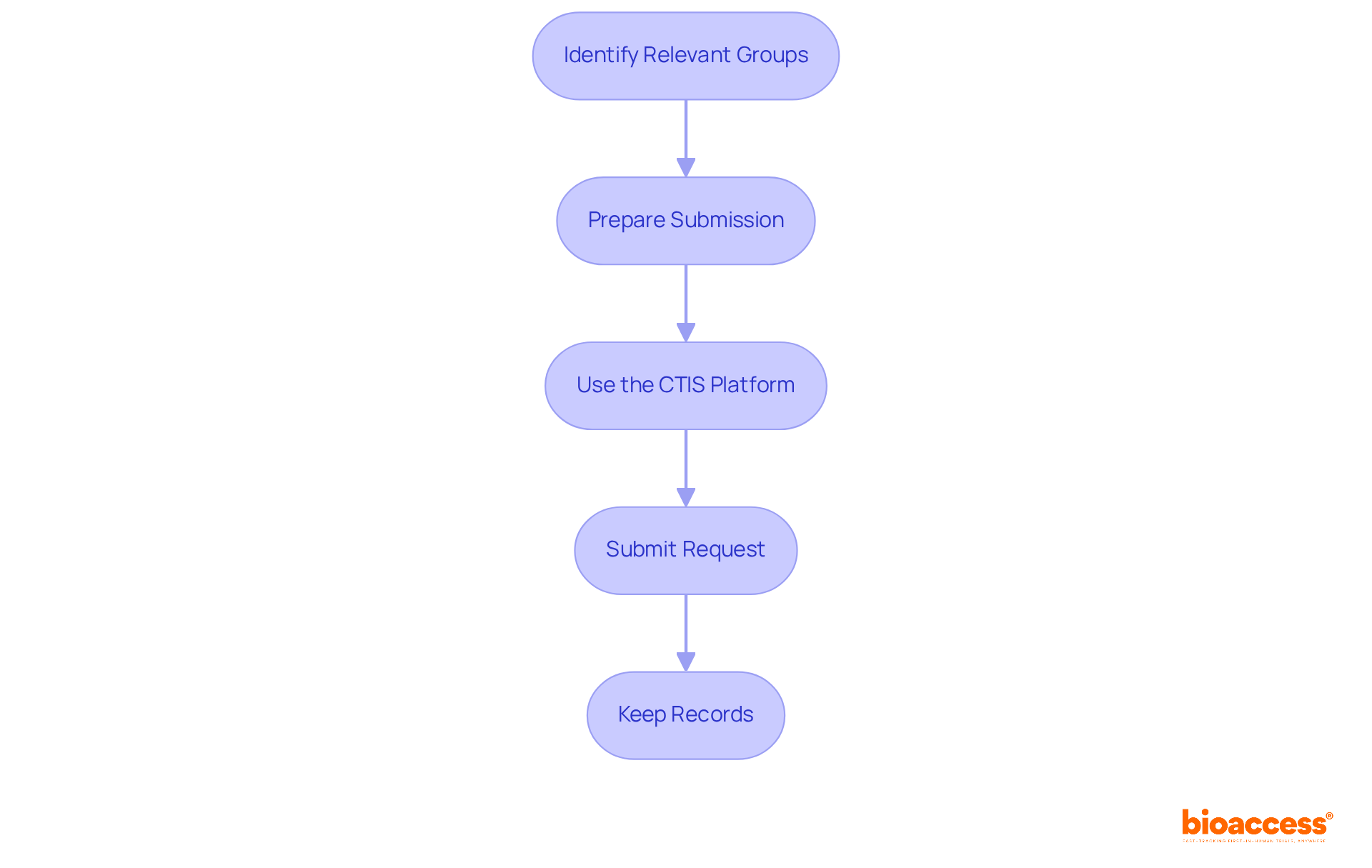
Once you have collected all required documentation, the next step is to submit your application for approval. Follow these steps:
Identify the Relevant Groups: Determine which ethics panels, including multicenter ethics approvals in Bulgaria and the Bulgarian Drug Agency (BDA), are relevant to your trial. Engaging with these committees early can enhance the relevance of your research outcomes.
Prepare Submission: Compile all documents in the required format, ensuring both electronic and hard copies of your proposal are ready. This preparation is crucial for a smooth submission process.
Use the CTIS Platform: Starting January 31, 2025, all submissions must be made through the Clinical Trials Information System (CTIS). Register on the platform and adhere to the submission guidelines to ensure compliance with the latest regulations.
Submit Request: Submit your request along with all required documents. Confirm receipt of your submission, as this acknowledgment is vital for tracking your progress.
Keep Records: Preserve copies of all submitted documents and communications with the review boards. This documentation is essential for future reference and compliance verification.
By adhering to these steps, you can manage the ethical review process efficiently, ensuring that your multicenter trial complies with the required moral standards and regulatory guidelines, particularly concerning multicenter ethics approvals in Bulgaria.
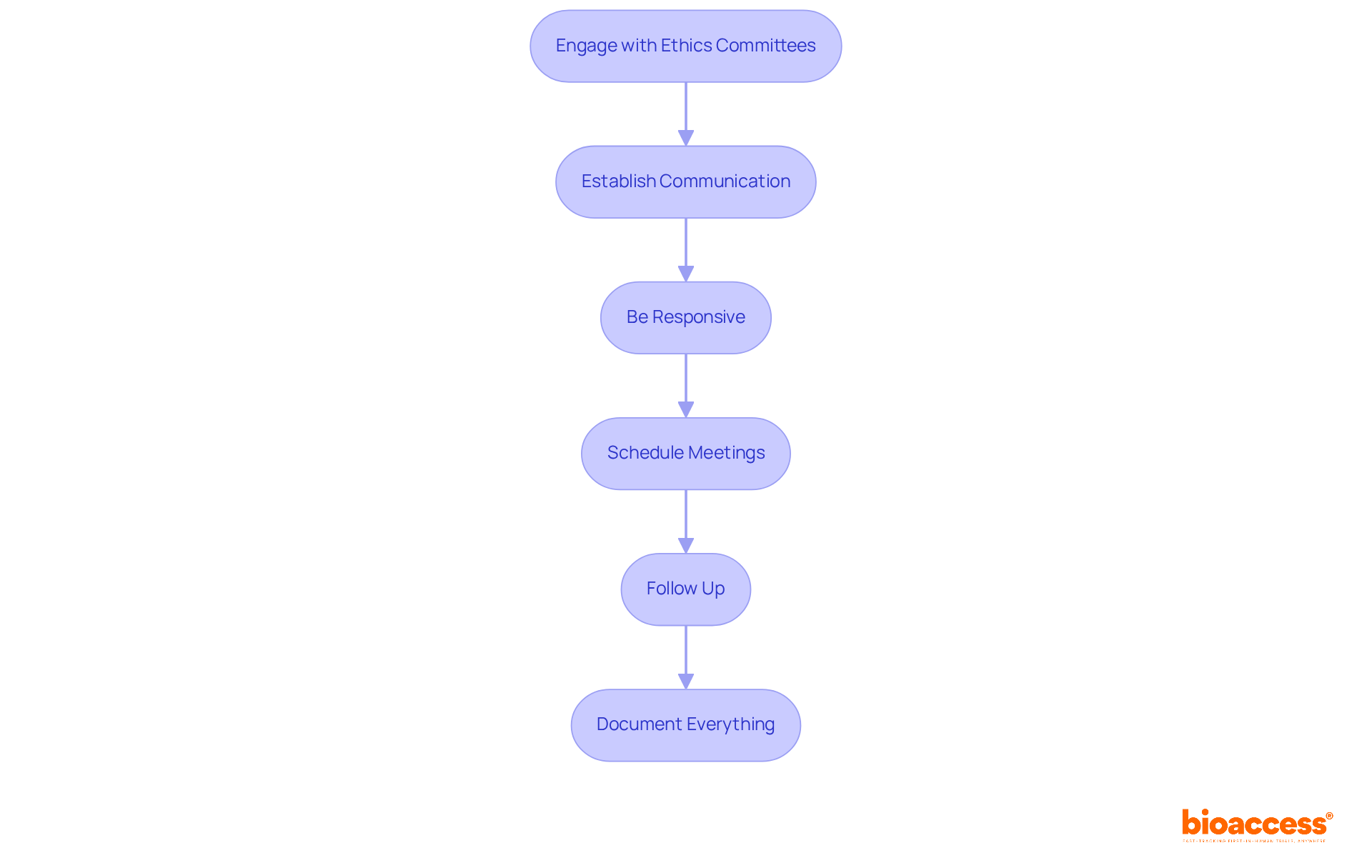
Interacting with review boards after your submission is crucial for securing prompt approvals in clinical research. Effective communication strategies can significantly enhance this process:
Statistics indicate that effective follow-up practices can enhance the likelihood of prompt approvals. Organizations that actively engage with review boards report improved outcomes. For example, research shows that patient-engaged studies achieve enrollment targets 25% faster and experience 40% fewer protocol amendments. By implementing these strategies, you can navigate the approval process more efficiently and cultivate a collaborative environment with ethics committees in Bulgaria.
Navigating the complex landscape of multicenter ethics approvals in Bulgaria is essential for the success of clinical research. A clear understanding of the regulatory framework, coupled with meticulous preparation and proactive engagement with ethics committees, lays the groundwork for obtaining timely approvals. This guide emphasizes that thorough knowledge of the EU Clinical Studies Regulation, alongside familiarity with the updated processes and documentation requirements, is crucial for researchers aiming to conduct studies in Bulgaria.
Key steps are outlined throughout the article, including:
By adhering to these guidelines, researchers can streamline their approval processes and mitigate common pitfalls that may lead to delays. The emphasis on ethical standards and participant safety further underscores the integrity of the research endeavor.
Ultimately, securing multicenter ethics approvals in Bulgaria is not just a regulatory requirement; it is a vital component of responsible research practice. By prioritizing ethical considerations and maintaining proactive communication with review boards, researchers can foster collaborative relationships that enhance the overall quality of clinical studies. As the landscape evolves in 2025, embracing these practices will not only facilitate smoother approvals but also contribute to the advancement of ethical clinical research in Bulgaria.
Why is understanding the regulatory framework for ethics approvals important in Bulgaria?
Understanding the regulatory framework is crucial for obtaining multicenter ethics approvals, which are necessary for successful clinical research in Bulgaria.
What regulation governs clinical studies in Bulgaria?
Bulgaria adheres to the EU Clinical Studies Regulation (EU No 536/2014), which outlines the requirements for conducting clinical studies and securing ethical approvals.
What role do ethics committees play in clinical studies in Bulgaria?
Ethics committees evaluate the ethical aspects of clinical studies, ensuring participant safety and compliance with ethical standards.
What changes are expected in the ethics committee structure in Bulgaria by 2025?
By 2025, Bulgaria will replace its former three-tier ethics committee system with a more efficient model that enhances oversight and accelerates the initiation of proceedings.
What are the legal requirements for conducting clinical research in Bulgaria?
Researchers must review legal requirements established by the Bulgarian Drug Agency (BDA) and the Ministry of Health, including necessary documentation like clinical research protocols and informed consent forms, as well as submission timelines.
How long is the targeted review duration for ethics approvals in Bulgaria?
The BDA has updated its regulations to target a review duration of approximately 35 days for ethics approvals.
What is the Clinical Trials Information System (CTIS) and its significance?
Starting January 31, 2025, all ongoing studies must comply with CTIS regulations, which aim to simplify submissions and improve overall study management across the EU.
What ethical standards must researchers uphold during the approval process?
Researchers must ensure informed consent and prioritize participant safety, which are essential for maintaining the integrity of their research and fostering a collaborative relationship with review boards.
How can researchers enhance their chances of obtaining prompt approvals in Bulgaria?
By effectively navigating the regulatory framework, including understanding ethics committee functions, legal requirements, and ethical standards, researchers can significantly enhance their chances of obtaining prompt approvals for clinical studies.