


The article emphasizes the critical importance of selecting appropriate theranostic clinical endpoints, a key factor in ensuring effective trials that seamlessly integrate therapeutic and diagnostic functions. It underscores best practices such as:
These strategies collectively enhance the relevance and reliability of study outcomes, particularly in the evolving landscape of patient care. By adopting these practices, clinical research can address key challenges and improve overall effectiveness, ultimately leading to better patient outcomes.
The integration of therapeutic and diagnostic functions in medicine has given rise to the concept of theranostic clinical endpoints, which are essential for evaluating treatment efficacy, particularly in oncology. This article delves into best practices for selecting these critical endpoints, offering insights that can enhance trial outcomes and patient care. As the landscape of medical research evolves, stakeholders must consider how to ensure their endpoint choices not only comply with regulatory standards but also resonate with patient needs and expectations.
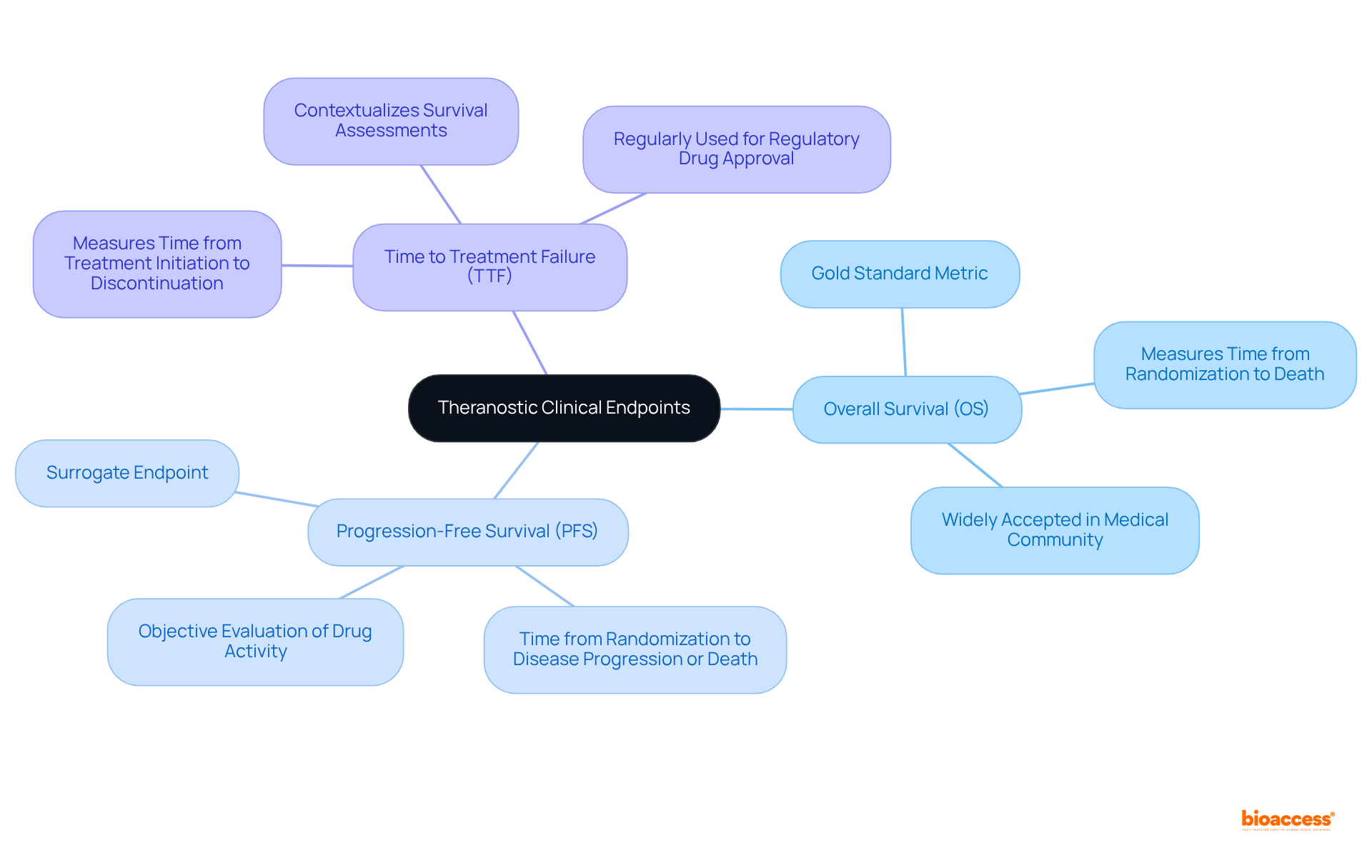
Theranostic medical targets are pivotal measures utilized to evaluate the efficacy of therapies that integrate both therapeutic and diagnostic functions. These targets play a crucial role in medical research, particularly in oncology, where they aid in assessing the effectiveness of theranostic approaches tailored to individual patient profiles. Commonly employed metrics include:
By establishing clear definitions for these focal points, researchers can design studies that yield meaningful data, ultimately guiding clinical decision-making through theranostic clinical endpoint selection guidance and enhancing patient outcomes. The importance of these targets is underscored by their ability to forge a direct connection between diagnostic imaging and therapeutic effectiveness, thereby refining treatment strategies.
As the field progresses in 2025, prioritizing precise outcome measures will be crucial for improving the efficiency of theranostic studies, guided by theranostic clinical endpoint selection guidance to meet the evolving demands of patient care and regulatory requirements. Additionally, the evolution of assessment measures, as highlighted in the case study 'Shift in Assessment Measures for Cancer Drug Evaluation,' emphasizes the increasing importance of OS and the need for deliberate measure selection to mitigate potential challenges in study design.
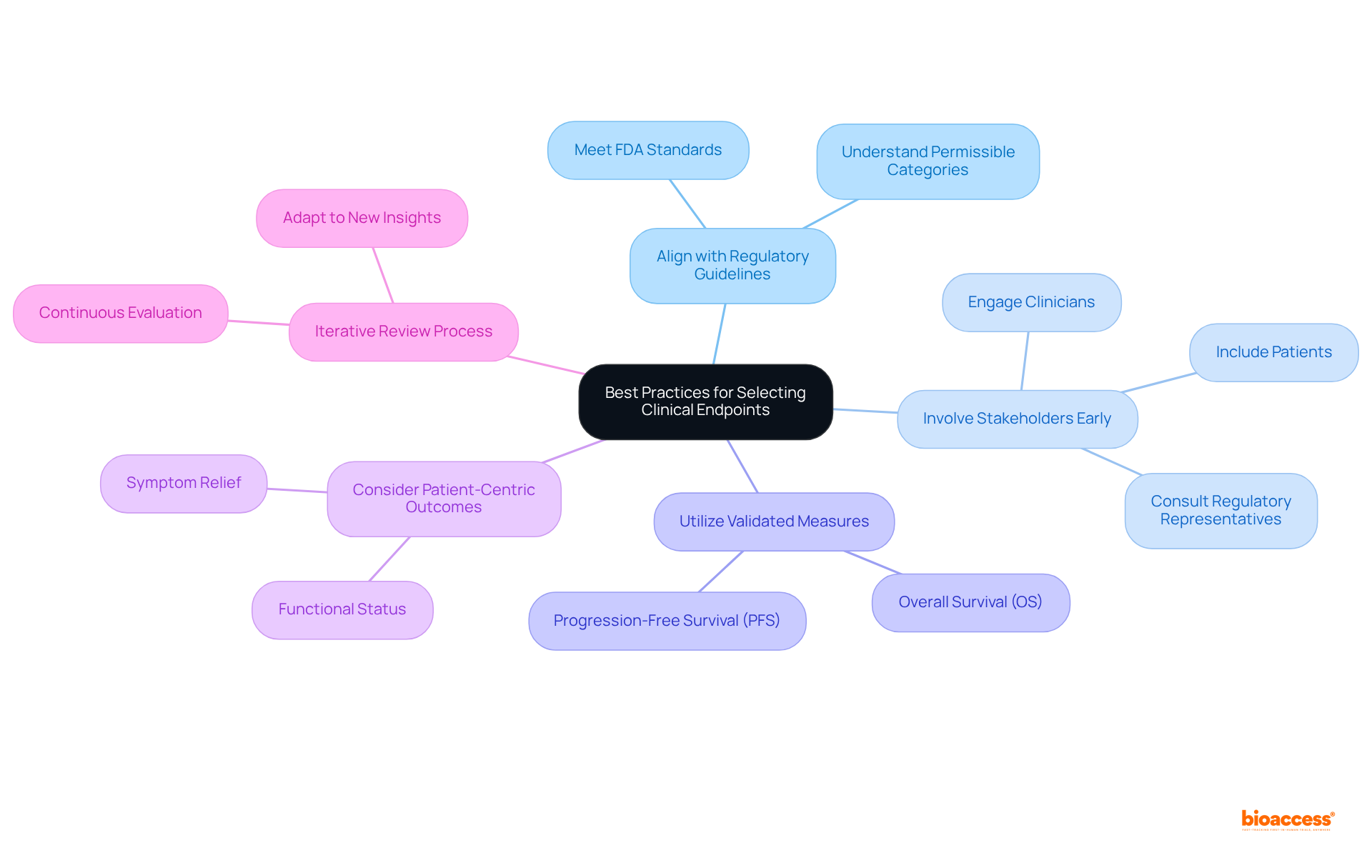
The theranostic clinical endpoint selection guidance emphasizes the necessity of adhering to several best practices when selecting clinical endpoints for theranostic trials to ensure successful outcomes.
Align with Regulatory Guidelines: It is imperative to ensure that the chosen targets meet the standards established by regulatory agencies such as the FDA. Comprehending the permissible categories of targets for particular therapeutic fields is essential for compliance and achieving positive study results.
Involve Stakeholders Early: Engaging key stakeholders—including clinicians, patients, and regulatory representatives—early in the outcome selection process is vital. Their perspectives are essential for recognizing targets that are both relevant and significant to the study's objectives.
Utilize Validated Measures: Selecting outcomes that have been validated in prior studies ensures reliability and reproducibility. Prioritizing widely accepted metrics, such as overall survival (OS) and progression-free survival (PFS), enhances the credibility of the study results.
Consider Patient-Centric Outcomes: Including measures that reflect patient experiences and quality of life, such as symptom relief and functional status, is crucial. This approach not only improves the significance of research outcomes but also aligns with the growing emphasis on patient-centered care in clinical studies.
Iterative Review Process: Establishing a consistent evaluation and enhancement procedure for selection criteria based on continuous research data and stakeholder input guarantees that the targets remain relevant and effective throughout the study's duration, adapting to any new insights or shifts in the therapeutic landscape.
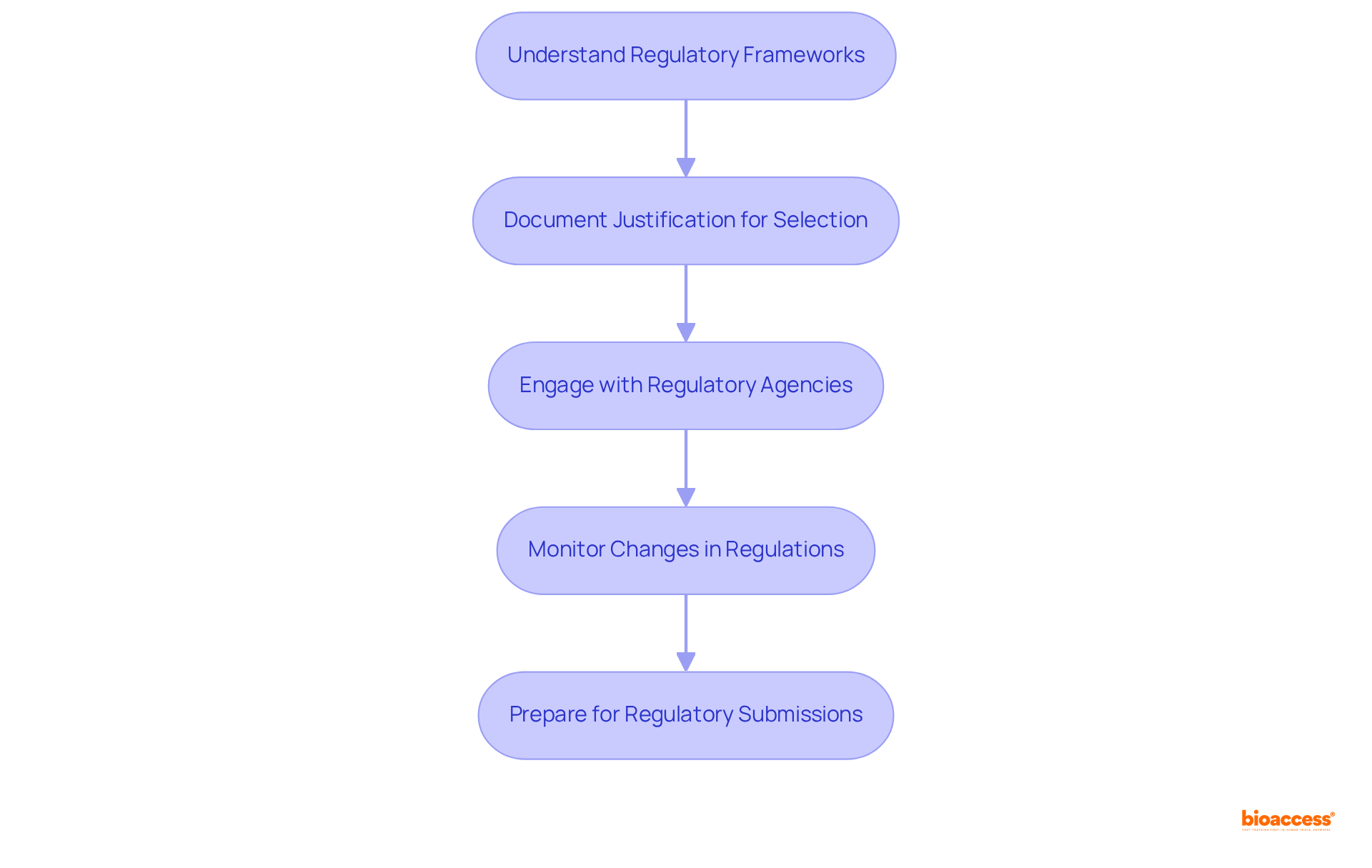
In selecting clinical endpoints for theranostic trials, it is essential to follow theranostic clinical endpoint selection guidance while effectively incorporating regulatory considerations. First, it is crucial to understand regulatory frameworks. Familiarize yourself with the guidelines from regulatory bodies such as the FDA and EMA concerning outcome determination. This encompasses recognizing suitable targets for different study types and ensuring conformity with regulatory expectations.
Next, document justification for selection choices. Clearly articulate the reasoning behind each selection in accordance with theranostic clinical endpoint selection guidance, detailing how they align with regulatory requirements and the overall objectives of the study. This documentation is vital for demonstrating compliance and supporting the integrity of the experiment.
Engaging with regulatory agencies early in the process is advisable. Discuss proposed objectives and gather feedback to recognize potential problems early, facilitating smoother execution. Additionally, monitoring changes in regulations is critical. Stay informed about updates to regulatory guidelines that may affect endpoint selection, as adapting to evolving standards is vital for maintaining compliance and ensuring the trial's relevance.
Finally, prepare for regulatory submissions by ensuring that the chosen targets are robustly backed by data and literature. This preparation can significantly enhance the likelihood of a successful approval process.
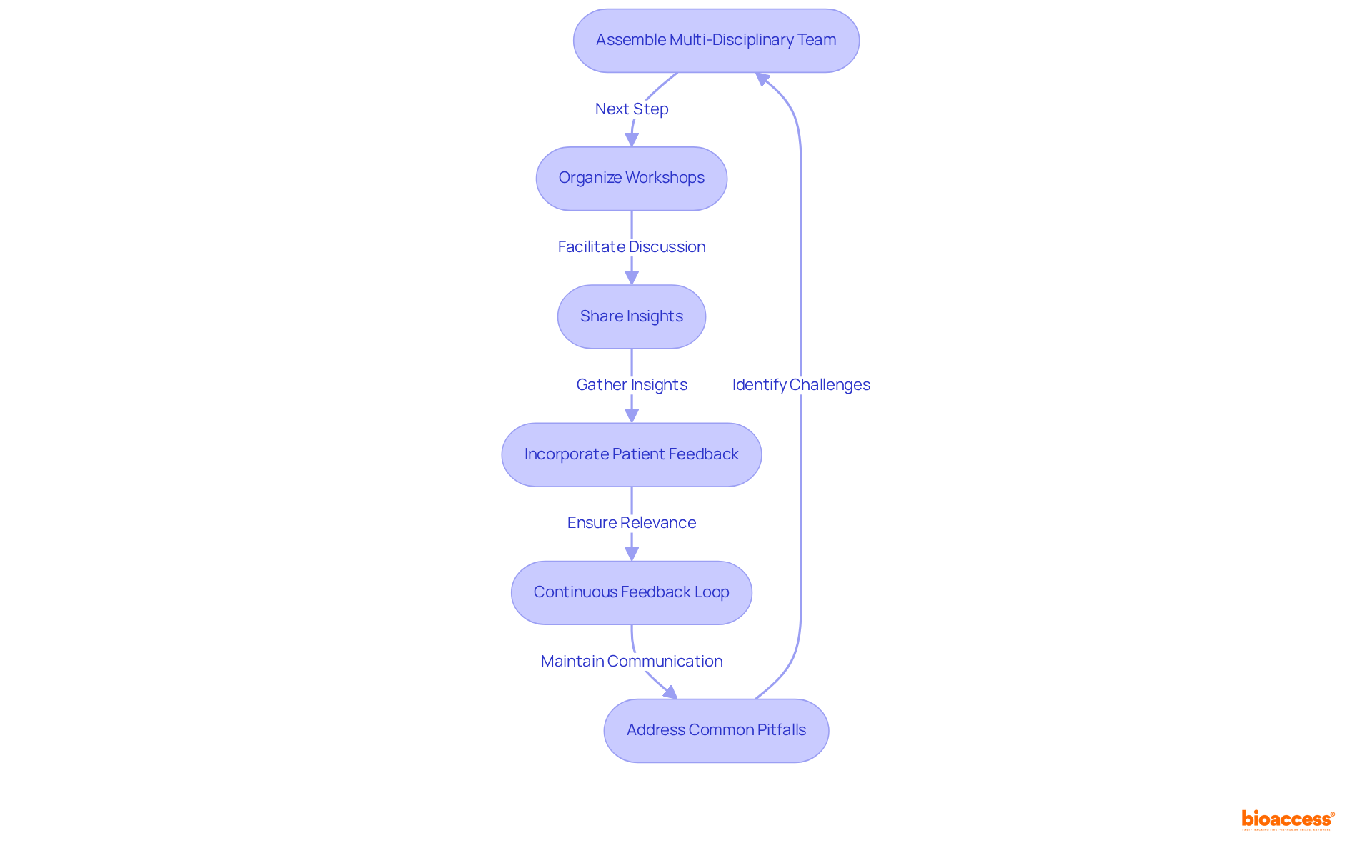
Collaboration among stakeholders is essential for effective theranostic clinical endpoint selection guidance in theranostic trials. To achieve this, it is critical to establish a multi-disciplinary team comprising clinicians, researchers, regulatory specialists, and patient representatives. This diverse group ensures a broad array of viewpoints is considered in outcome determination, fostering innovative thinking and comprehensive decision-making.
Organizing workshops and meetings facilitates discussions on terminal options, allowing stakeholders to share insights and reach a consensus. This collaborative approach can lead to innovative solutions and a cohesive vision for selection. Actively seeking and incorporating patient feedback regarding the outcomes that matter most to them guarantees that these outcomes are not only scientifically valid but also relevant and significant from the patient's perspective, thereby increasing the study's impact.
Encouraging the exchange of data and insights from prior experiments among stakeholders can significantly enhance the quality of decisions made concerning endpoint selection. Drawing on lessons learned from past experiences is invaluable. Furthermore, implementing a continuous feedback loop establishes ongoing communication among stakeholders throughout the testing process, enabling adjustments to endpoints as new information arises, ensuring alignment with study goals and responsiveness to participant needs.
For instance, strategic partnerships and acquisitions represented 65% of total investment activity in the research market in 2023, underscoring the significance of collaboration in achieving successful results. The 'Collaborate Forward Initiative' serves as a practical illustration of successful collaboration in health research, aiming to develop a guide for effective cross-stakeholder cooperation in medical studies.
As Mike Clay, Senior Vice President of Global Project Delivery, states, "We believe that collaboration with clinical research sites is key to unlocking efficiencies and productivity gains that will streamline the clinical trial process." However, while collaboration is crucial, it is also important to be aware of common pitfalls, such as miscommunication and lack of alignment on goals, which can hinder the collaboration process. Identifying and addressing these challenges can provide valuable insights for theranostic clinical endpoint selection guidance.
The selection of theranostic clinical endpoints is paramount in the design and execution of effective clinical trials, especially within the rapidly evolving field of oncology. Understanding the significance of these endpoints enables researchers to enhance the relevance and impact of their studies, ensuring that therapeutic and diagnostic measures align with patient needs and regulatory standards.
Key arguments presented emphasize the necessity of adhering to best practices, such as:
These practices not only promote compliance and enhance the credibility of the research but also foster collaboration among diverse stakeholders—an essential element for achieving meaningful results in theranostic trials.
Ultimately, the importance of selecting appropriate clinical endpoints cannot be overstated. As the landscape of medical research continues to evolve, embracing these guidelines and fostering a collaborative approach will be vital for driving innovation and improving patient outcomes. Stakeholders are encouraged to prioritize these practices to navigate the complexities of theranostic trials effectively, ensuring that the future of personalized medicine is both impactful and patient-focused.
What are theranostic clinical endpoints?
Theranostic clinical endpoints are pivotal measures used to evaluate the efficacy of therapies that integrate both therapeutic and diagnostic functions, particularly in medical research and oncology.
Why are theranostic clinical endpoints important?
They are important because they help assess the effectiveness of theranostic approaches tailored to individual patient profiles, guiding clinical decision-making and enhancing patient outcomes.
What are common metrics used as theranostic clinical endpoints?
Commonly employed metrics include overall survival (OS), progression-free survival (PFS), and time to treatment failure (TTF).
What is overall survival (OS) and why is it significant?
Overall survival (OS) is often regarded as the 'gold standard' primary metric in oncology, providing a crucial measure of the effectiveness of cancer treatments.
How does progression-free survival (PFS) contribute to theranostic evaluations?
Progression-free survival (PFS) provides essential insights into the impacts of theranostic agents, helping to evaluate their effectiveness over time.
What role does time to treatment failure (TTF) play in theranostic studies?
Time to treatment failure (TTF) is significant for evaluating the efficacy of treatments within the theranostic framework, indicating how long a treatment remains effective before it fails.
How will the evolution of assessment measures impact theranostic studies by 2025?
The evolution of assessment measures will be crucial for improving the efficiency of theranostic studies, ensuring that outcome measures meet the evolving demands of patient care and regulatory requirements.
What challenges might arise in the design of theranostic studies?
Potential challenges may include the deliberate selection of measures to ensure meaningful data, as highlighted by recent case studies emphasizing the importance of overall survival and other metrics.