


Understanding antibody isotypes is crucial for the success of clinical research. Each isotype—IgM, IgD, IgG, IgA, and IgE—has distinct structural and functional roles that significantly influence therapeutic efficacy and study design. Recognizing these differences enables researchers to tailor their approaches effectively, enhancing treatment outcomes and optimizing clinical trial strategies. Ultimately, this knowledge leads to the development of more effective therapies.
Understanding the intricate world of antibody isotypes is crucial for advancing clinical research and therapeutic strategies.
With five principal forms—IgM, IgD, IgG, IgA, and IgE—each isotype plays a unique role in the immune response, influencing everything from disease susceptibility to treatment efficacy.
The challenge, however, lies in determining how these distinct characteristics can be leveraged to optimize clinical outcomes.
What insights can researchers gain from a deeper exploration of antibody isotypes to enhance the design and success of their studies?
This exploration is not just academic; it is essential for driving innovation in therapeutic approaches.
Immunoglobulin isotypes, also known as classes of immunoglobulins, represent the various forms of antibody isotypes produced by B cells in response to antigens. Humans have five principal antibody isotypes, which are IgM, IgD, IgG, IgA, and IgE. Each antibody isotype possesses unique structural and functional characteristics that dictate its role in the immune response.
For example, IgG is the most prevalent immunoglobulin in serum and plays a vital role in long-term immunity, whereas IgE is predominantly associated with allergic reactions. Understanding these distinctions is essential for clinical research, as the choice of immune protein type can significantly influence the efficacy of therapeutic interventions and the design of clinical trials.
By recognizing the specific functions of each variant, researchers can more effectively tailor their strategies to enhance patient outcomes and increase the success rates of new therapies.
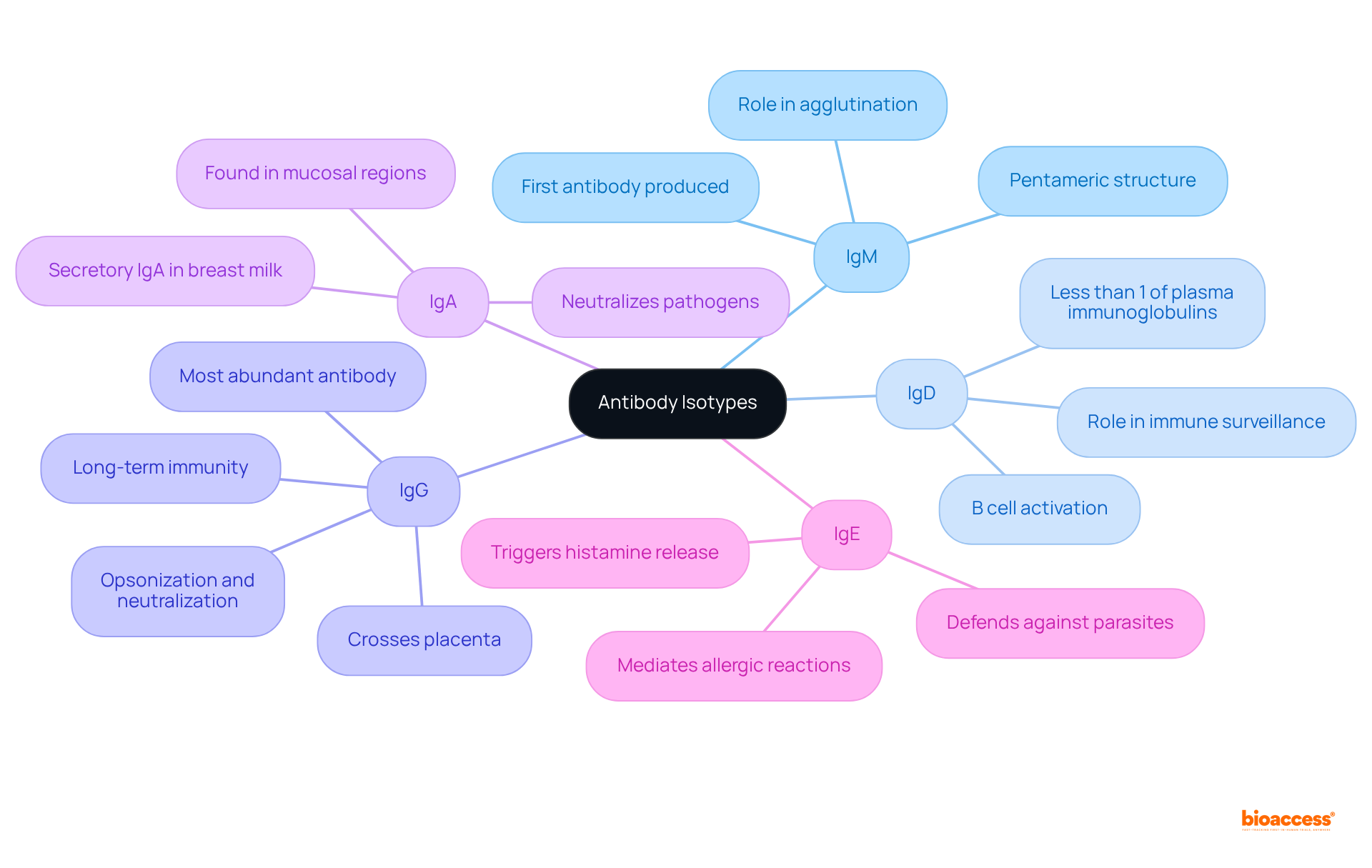
Each antibody isotype possesses distinct structural features that dictate its specific functions in the immune response:
IgM: As the largest antibody isotype, IgM is typically the first antibody produced during the primary immune response to an infection. Its pentameric structure allows it to effectively bind multiple antigens, enhancing its ability to activate complement pathways and agglutinate pathogens, making it essential for early immune defense.
IgD: Primarily located on the surface of B cells, IgD's precise functions are still being elucidated. However, it is known to serve as a B-cell antigen receptor and plays a significant role in B cell activation, maturation, and regulation, contributing to the overall immune response.
IgG: The most abundant antibody in serum, comprising about 75% of antibodies, IgG is vital for opsonization, neutralization of pathogens, and facilitating antibody-dependent cellular cytotoxicity (ADCC). It comprises four subclasses (IgG1, IgG2, IgG3, IgG4), each exhibiting varying capacities to activate complement and bind to Fc receptors, thus influencing therapeutic strategies. Notably, IgG can cross the placenta, providing passive immunity to the fetus.
IgA: Predominantly found in mucosal areas and secretions such as saliva and breast milk, IgA exists as monomers in blood and dimers in secretions, connected by a J chain. It is crucial for mucosal immunity, preventing pathogen adherence to mucosal surfaces and thereby playing a key role in protecting against infections in the gastrointestinal and respiratory tracts. Recent advancements in secretory IgA research are improving knowledge on mucosal vaccines targeting respiratory and gastrointestinal infections.
IgE: This isotype is primarily involved in allergic reactions and defense against parasitic infections. IgE is present at concentrations roughly 10,000 times lower than those of IgG in healthy individuals. It binds to allergens and triggers mast cell degranulation, leading to the release of histamine and other inflammatory mediators, which are critical in the body's response to allergens. IgE-targeted therapies are progressing in treating allergic diseases by blocking IgE interactions and reducing symptoms.
Comprehending these traits is crucial for researchers, as they greatly affect the choice of agents and antibody isotypes for medical applications and clinical studies.
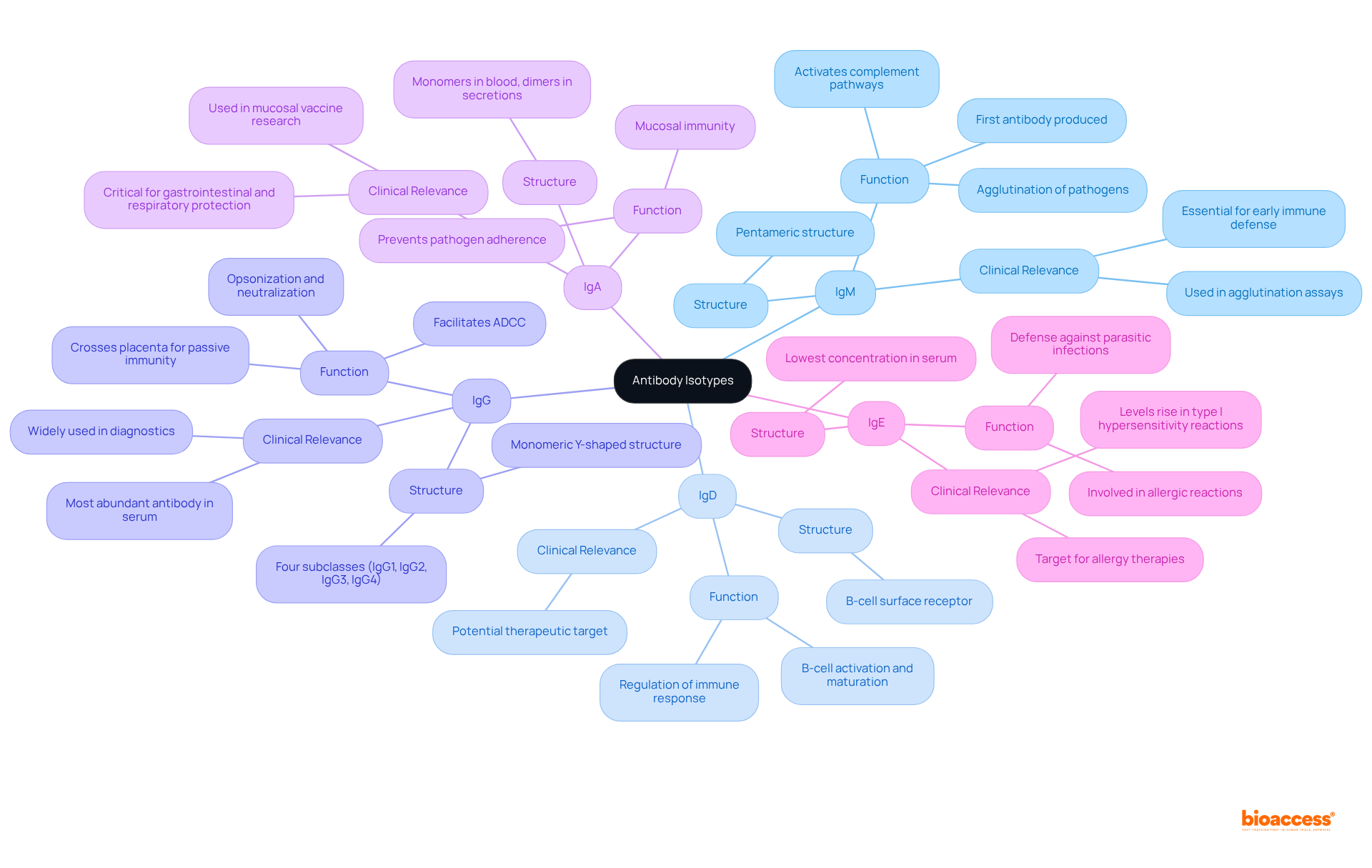
The choice of immune response type is crucial in assessing treatment effectiveness and influencing clinical research outcomes. For example, IgG is the most commonly utilized isotype in therapeutic antibodies, renowned for its capacity to elicit robust immune responses and its extended half-life, which makes it particularly effective for managing chronic conditions. As noted by Stephen A. Beers, 'isotype choice is vital to treatment success with agents that work through different mechanisms.' In contrast, IgM plays a pivotal role during early-stage infections, as it is produced rapidly and can form complexes that enhance the clearance of pathogens, thereby ensuring effective treatment outcomes. Meanwhile, IgA is essential for mucosal immunity, especially in vaccines targeting the gut and respiratory tract, providing localized protection that can significantly influence clinical trial endpoints. Lastly, IgE is being explored for its potential in managing allergic conditions, where its role in allergic reactions can be harnessed to alleviate symptoms, underscoring its clinical significance.
In the context of clinical research, a comprehensive understanding of these isotype-specific impacts is vital for optimizing study designs, selecting appropriate endpoints, and ultimately advancing the development of more effective therapies. For instance, the capacity of IgG to facilitate robust immune reactions and its extended half-life can lead to quicker enrollment in clinical trials, as demonstrated by bioaccess®'s ability to obtain ethical approvals in 4-6 weeks and achieve enrollment that is 50% swifter than conventional markets. Researchers must carefully consider the distinct characteristics of each variant when designing clinical trials to ensure alignment with the intended treatment mechanisms and desired immune responses. Additionally, insights from case studies on the influence of immunoglobulin isotype on therapeutic antibody function can provide practical examples that reinforce these theoretical points.
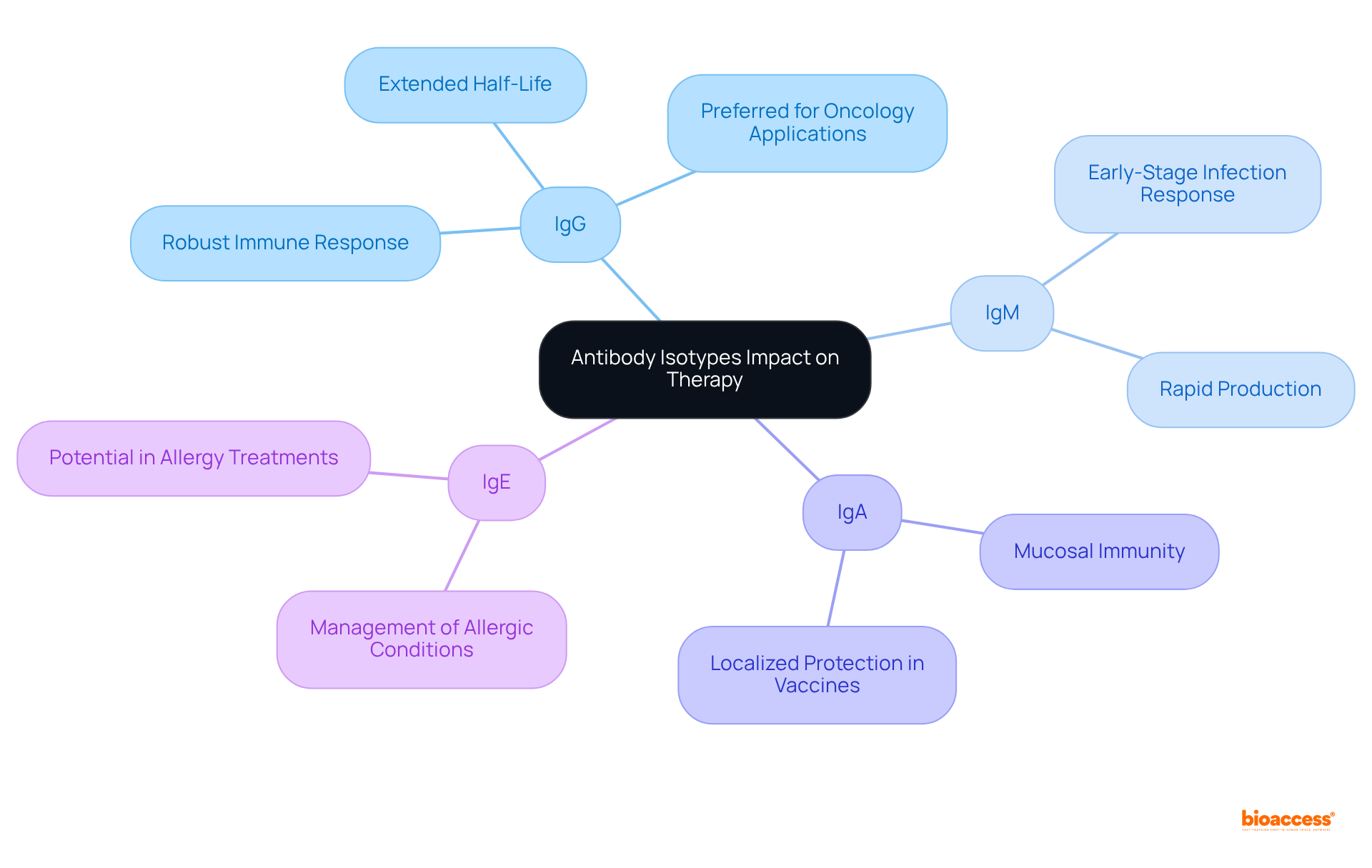
Understanding the various antibody isotypes is fundamental for achieving success in clinical research. Each isotype—IgM, IgD, IgG, IgA, and IgE—plays a distinct role in the immune response, influencing both therapeutic efficacy and the design of clinical trials. By recognizing these differences, researchers can tailor their approaches to improve patient outcomes and enhance the effectiveness of new treatments.
The article delves into the structural and functional characteristics of each antibody isotype, highlighting their unique contributions to the immune system. For instance, IgG's prominence in therapeutic applications is underscored by its robust immune response capabilities, while IgA’s role in mucosal immunity is critical for vaccine development. Additionally, the rapid response of IgM during infections and the specialized functions of IgE in allergic reactions further illustrate the importance of selecting the appropriate isotype for specific clinical applications.
In conclusion, the significance of antibody isotypes in clinical research cannot be overstated. A thorough understanding of these immunoglobulins not only informs the selection of therapeutic agents but also enhances the design and execution of clinical trials. As research continues to evolve, prioritizing the study of antibody isotypes will be crucial in advancing therapeutic strategies and improving patient care. Embracing this knowledge is essential for researchers aiming to navigate the complexities of immunology and maximize the impact of their work in clinical settings.
What are antibody isotypes?
Antibody isotypes, or classes of immunoglobulins, are the various forms of antibodies produced by B cells in response to antigens.
How many principal antibody isotypes do humans have?
Humans have five principal antibody isotypes: IgM, IgD, IgG, IgA, and IgE.
What is the significance of understanding different antibody isotypes in clinical research?
Understanding the distinctions between antibody isotypes is essential in clinical research because the choice of immune protein can significantly influence the efficacy of therapeutic interventions and the design of clinical trials.
What role does IgG play in the immune response?
IgG is the most prevalent immunoglobulin in serum and plays a vital role in long-term immunity.
What is the association of IgE in the immune response?
IgE is predominantly associated with allergic reactions.
How can knowledge of antibody isotypes enhance patient outcomes?
By recognizing the specific functions of each antibody isotype, researchers can tailor their strategies more effectively, which can enhance patient outcomes and increase the success rates of new therapies.