


Case Report Forms (CRFs) are standardized documents that play a pivotal role in the systematic collection of patient data within clinical research. Their significance lies in ensuring both accuracy and compliance with regulatory standards. By detailing how CRFs facilitate consistent data collection, reduce errors, and enhance operational efficiency, the article underscores their essential contribution to achieving more reliable research outcomes.
Case Report Forms (CRFs) are integral to the field of clinical research, acting as the cornerstone for data collection that drives medical progress. By offering a standardized approach to gathering essential patient information, these forms guarantee that research remains both reliable and compliant with regulatory standards. As the landscape of clinical trials evolves, one must consider: how do CRFs adapt to meet the growing demands for efficiency and accuracy? This article explores the definition, significance, and essential features of CRFs, tracing their transformative journey from paper to digital formats and underscoring their critical role in shaping the future of clinical research.
A Case Report Form (CRF) is an essential standardized document used in research studies to systematically gather information from each participating patient through case report forms. Available in both paper-based and electronic formats, case report forms are meticulously designed to capture all protocol-required information, ensuring that the data collected is both consistent and reliable. They serve as the primary tool for investigators to document critical patient information, treatment outcomes, and any adverse events that may arise during the study. This structured methodology is vital for evaluating the safety and efficacy of medical interventions.
The significance of case report forms is further emphasized by case studies that showcase their role in successful medical investigations. For instance, bioaccess® has effectively leveraged the regulatory efficiency of Latin America to facilitate early-stage trials, achieving ethical approvals in a mere 4-6 weeks, which allows for enrollment that is 50% quicker than in conventional markets. Furthermore, the diverse patient groups in the Balkans have enhanced recruitment initiatives, demonstrating how clinical research facilities contribute to a more efficient and effective enrollment process in early-phase studies. As Heinz R. Pagels observed, "Science cannot resolve moral conflicts, but it can assist in more precisely framing the discussions surrounding these conflicts," emphasizing the ethical dimensions that trial frameworks must address in scientific inquiry.
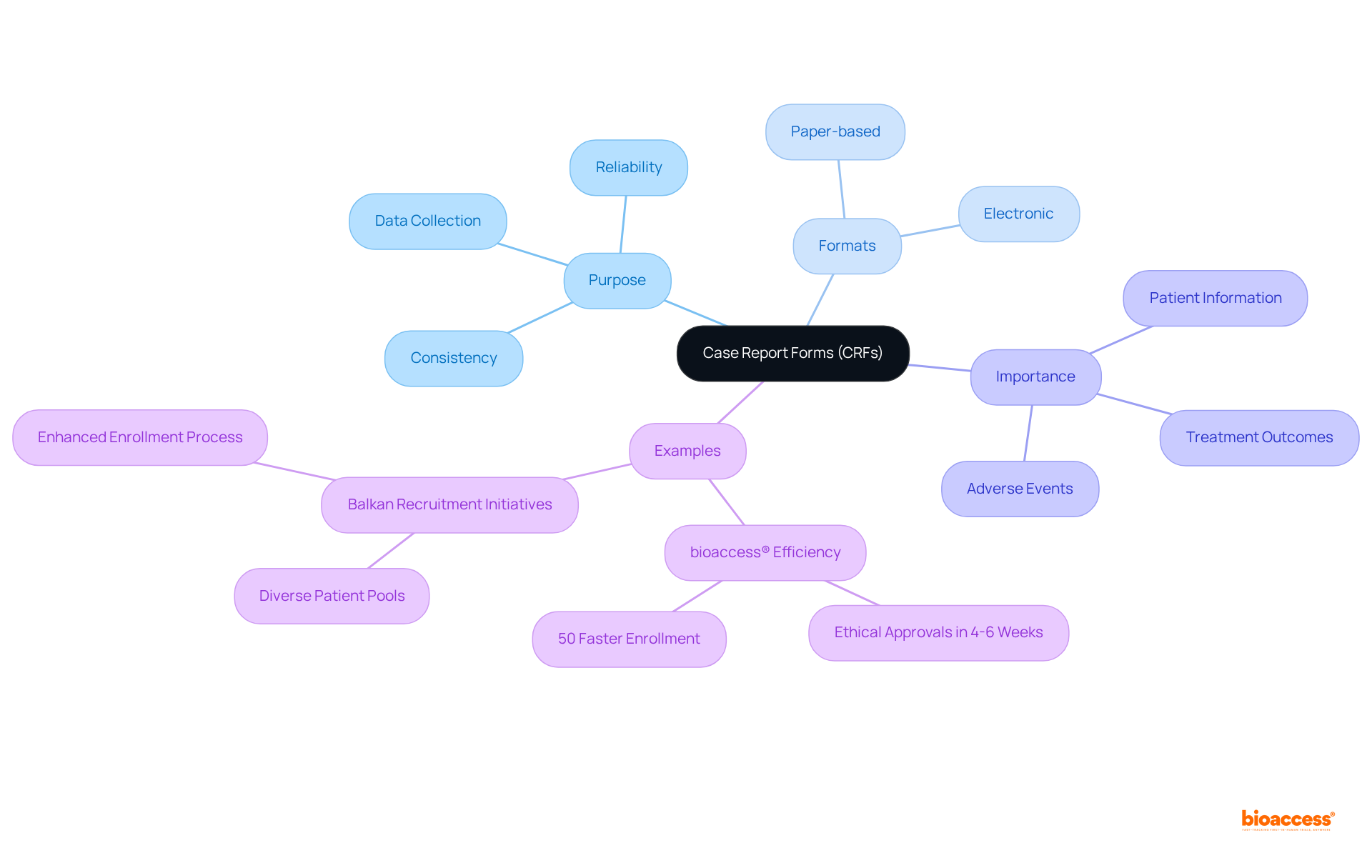
Case report forms are essential instruments in clinical research, offering a structured framework for information collection that is crucial for both regulatory submissions and scientific evaluation. They ensure the consistent capture of necessary information across various locations and participants by utilizing case report forms, thereby playing a crucial role in maintaining the validity of trial results.
The uniformity in information gathering through case report forms significantly decreases the chances of errors and inconsistencies, thereby enhancing the trustworthiness of results. Regulatory agencies mandate thorough documentation, making case report forms essential for compliance with Good Clinical Practice (GCP) guidelines, which are based on 14 fundamental principles.
Indeed, the use of uniform case report forms has been shown to expedite patient recruitment and decrease the time to market for new therapies, underscoring their importance in the research landscape. Furthermore, organizations that effectively utilize case report forms can streamline their information management processes—including gathering, refining, and archiving data—to meet the stringent requirements of regulatory submissions, ultimately resulting in more favorable outcomes in research trials.
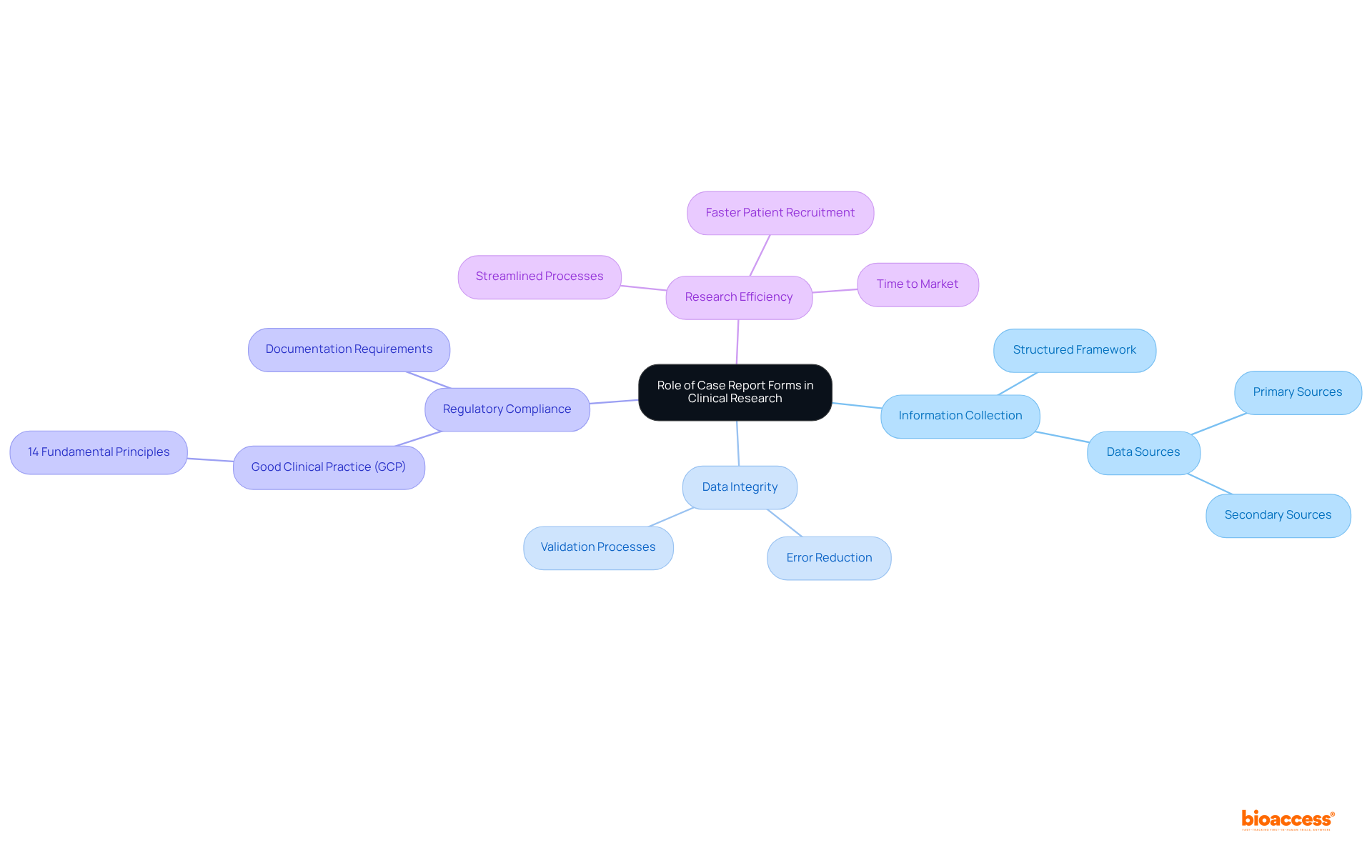
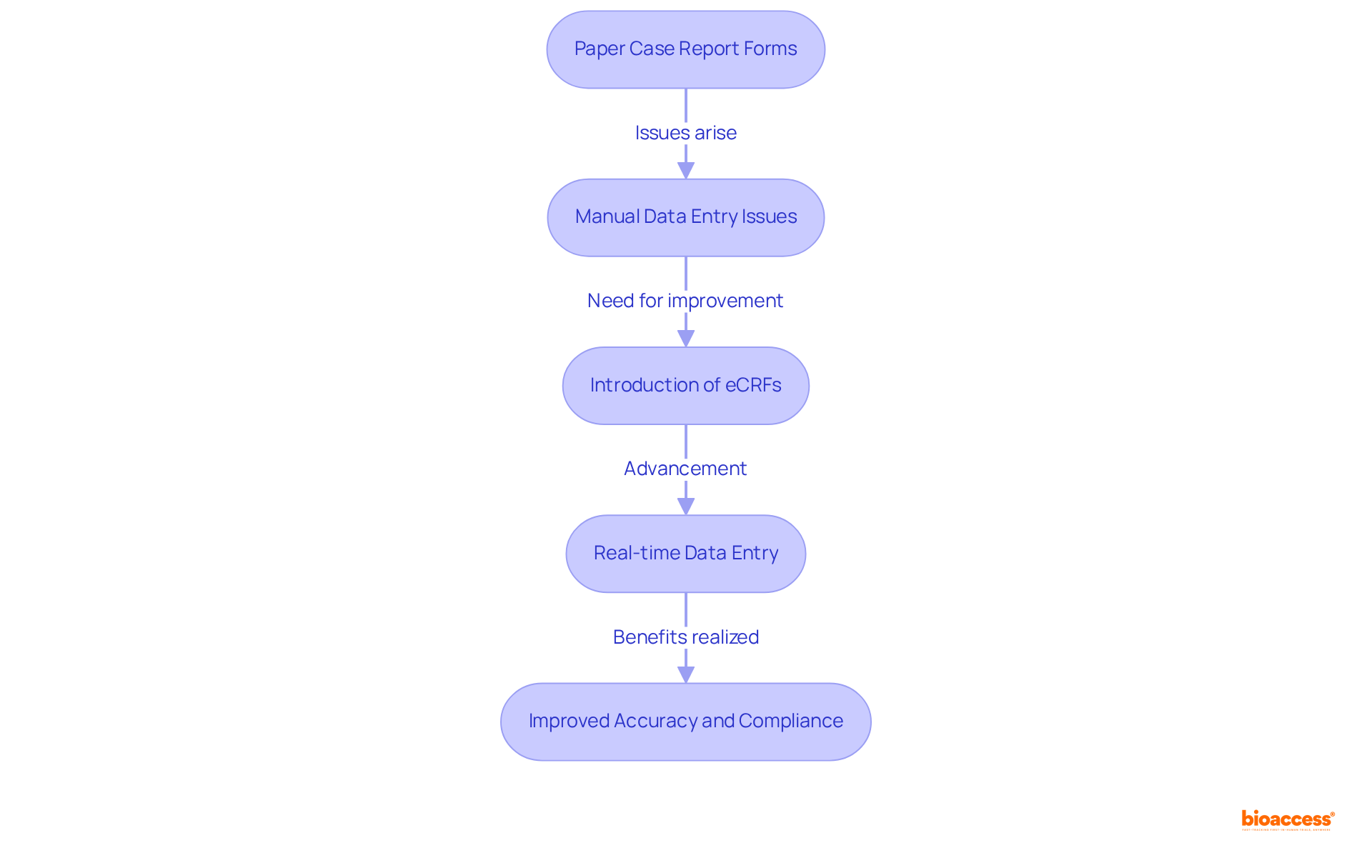
Historically, case report forms were on paper, necessitating manual entry of information, which often resulted in inconsistencies and errors. The advent of electronic case report forms (eCRFs) marks a significant advancement in clinical research, streamlining the processes of information collection and management.
Case report forms facilitate real-time data entry, substantially reducing the risk of transcription errors while enabling more efficient monitoring and analysis. This transition to digitization not only accelerates the pace of information gathering but also enhances the accuracy of case report forms and ensures compliance with regulatory standards.
Notably, studies indicate that the implementation of case report forms can lead to a 30% reduction in management costs, underscoring their effectiveness in boosting operational efficiency. Furthermore, a Phase III influenza vaccine study demonstrated that 85% of parents preferred utilizing their own devices for eDiary entries, showcasing the favorable impact of user-friendly case report forms on enrollment and operational workflows.
As the clinical research landscape continues to advance, the integration of technology into information capture processes is crucial for achieving high-quality outcomes and sustaining competitiveness in a market projected to surpass $70 billion by 2025.
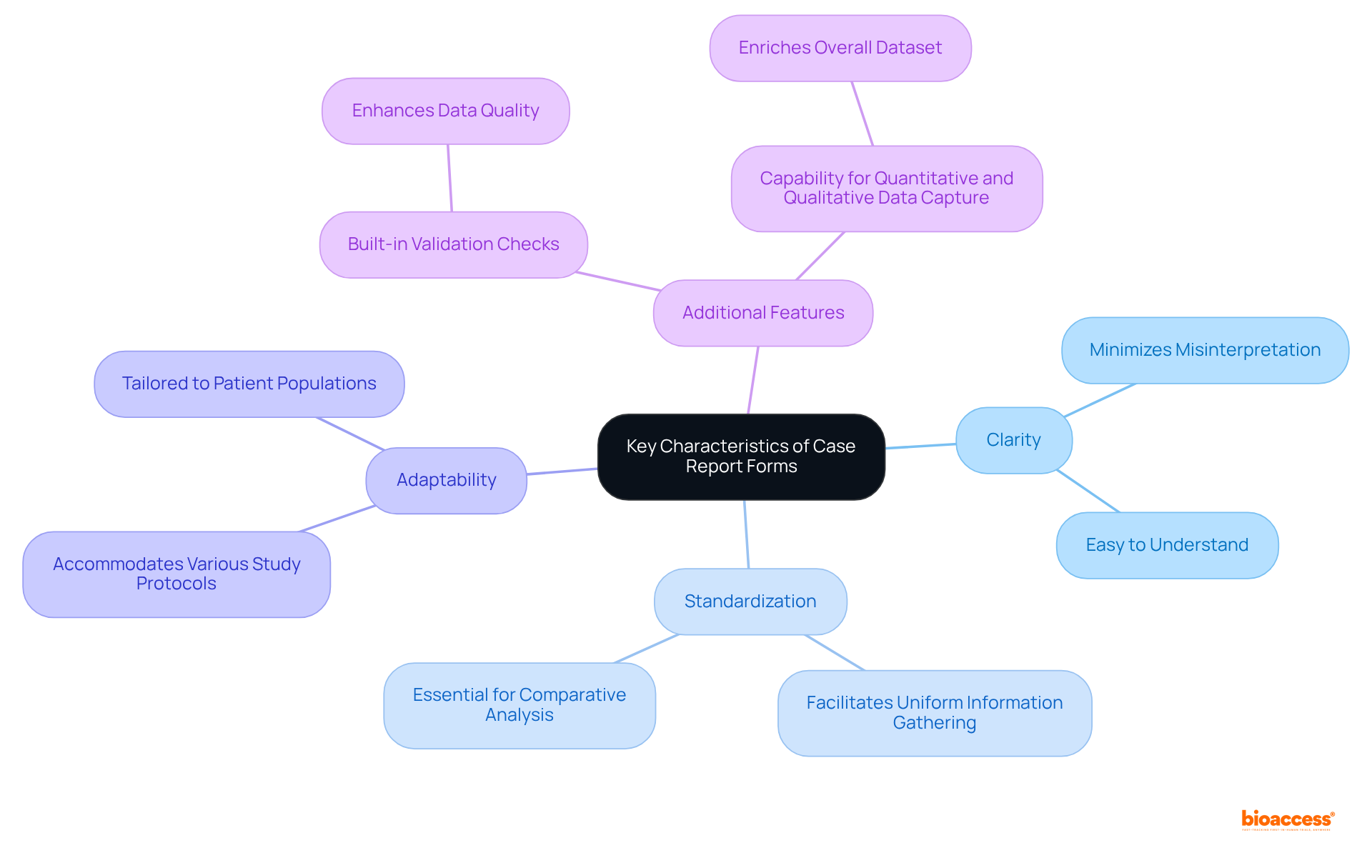
Effective case report forms (CRFs) are defined by clarity, standardization, and adaptability, which are essential for successful clinical research. Clarity guarantees that questions and fields are easily understood by all users, thereby minimizing the risk of misinterpretation. Standardization across case report forms facilitates uniform information gathering, which is an essential component for comparative analysis. Furthermore, adaptability allows case report forms to accommodate various study protocols and patient populations, ensuring they can be tailored to meet specific research needs.
Additional critical features include:
Case Report Forms (CRFs) are the cornerstone of clinical research, providing a structured and standardized approach to collecting essential patient data. Their importance cannot be overstated; they ensure the reliability and consistency of information gathered across diverse study populations, ultimately bolstering the integrity of clinical trials.
This article has underscored the crucial role of CRFs in enhancing data accuracy, expediting patient recruitment, and ensuring compliance with regulatory standards. The transition from traditional paper forms to electronic formats represents a significant advancement, facilitating real-time data entry and reducing management costs. Moreover, key characteristics such as clarity, standardization, and adaptability are vital for effective CRF design, ensuring they cater to the varied needs of clinical research.
Given these insights, it is evident that the effective utilization of case report forms is paramount for the success of clinical trials. As the landscape of medical research continues to evolve, it is essential for researchers to embrace advanced CRF technologies and best practices to deliver safe and effective therapies to the market efficiently. Acknowledging the significance of CRFs in data collection and management empowers stakeholders to uphold the highest standards of clinical research, ultimately enhancing patient outcomes.
What is a Case Report Form (CRF)?
A Case Report Form (CRF) is a standardized document used in research studies to systematically gather information from participating patients. It can be in paper-based or electronic formats and is designed to capture all protocol-required information, ensuring the data collected is consistent and reliable.
What is the purpose of Case Report Forms in research studies?
CRFs serve as the primary tool for investigators to document critical patient information, treatment outcomes, and any adverse events during a study. This structured methodology is essential for evaluating the safety and efficacy of medical interventions.
How do Case Report Forms contribute to medical investigations?
The significance of CRFs is highlighted by case studies that demonstrate their role in successful medical investigations. They help ensure that data is collected systematically, which is crucial for the integrity of clinical research.
Can you provide an example of the effectiveness of CRFs in clinical trials?
An example includes bioaccess® utilizing the regulatory efficiency of Latin America to facilitate early-stage trials, achieving ethical approvals in 4-6 weeks, which allows for enrollment that is 50% quicker than in conventional markets.
How do diverse patient groups impact the recruitment process in clinical studies?
Diverse patient groups, such as those in the Balkans, have enhanced recruitment initiatives, demonstrating how clinical research facilities contribute to a more efficient and effective enrollment process in early-phase studies.
What ethical considerations are associated with trial frameworks in scientific inquiry?
Ethical dimensions in trial frameworks are important, as observed by Heinz R. Pagels, who stated that while science cannot resolve moral conflicts, it can help frame discussions surrounding these conflicts more precisely.