


CCDS Medical Certification is essential for ensuring compliance with established medical research standards, thereby enhancing the reliability of data collected during clinical trials and improving patient outcomes. This certification not only streamlines research processes and reduces errors but also positions organizations favorably in a competitive market. Ultimately, it fosters a culture of excellence in clinical research, highlighting the critical need for organizations to prioritize this certification to remain at the forefront of the industry.
The landscape of clinical research is increasingly defined by the need for standardized practices that ensure data integrity and patient safety. At the heart of this evolution lies the Clinical Standards Medical Certification (CCDS), a crucial accreditation that not only enhances the reliability of clinical trials but also positions organizations favorably in a competitive market. As the demand for harmonized information grows, how can entities leverage CCDS certification to improve their operational efficiency and ultimately drive better patient outcomes?
Clinical Standards Medical Certification represents a formal acknowledgment of compliance with established medical research standards. This accreditation is vital for ensuring that medical information is collected, managed, and disclosed in accordance with regulatory standards and industry best practices.
For entities engaged in medical trials, the approval of data standards significantly enhances the reliability of the information produced, which is essential for successful regulatory submissions. Particularly, Medtech, Biopharma, and Radiopharma firms benefit from this accreditation as it underscores their commitment to quality and adherence in research.
By securing this accreditation, these organizations not only optimize their operational efficiency but also position themselves favorably in a competitive market, ultimately leading to improved patient outcomes and increased confidence in their innovations.
bioaccess® offers comprehensive trial management services, including:
All of which are crucial for obtaining necessary approvals. The costs associated with acquiring these credentials vary, with ACDIS members contributing $280 and non-members $380, making it a worthwhile investment for organizations seeking to enhance their operational efficiency and confidence in their innovations.
In the field of clinical research, CCDS certification plays a crucial role in ensuring that information gathered during trials conforms to standardized formats and protocols. This standardization is especially vital in a globalized research environment where data from diverse regions must be harmonized. Current trends indicate a significant shift towards information harmonization, with organizations increasingly recognizing the importance of standardized credentials to enhance their research methods and outcomes. Numerous prominent biopharma firms have reported improved trial quality and efficiency after adopting CCDS standards, demonstrating the tangible benefits of this certification in fostering a culture of excellence in research. As Frank Pétavy noted, "Standardization helps build efficient and interoperable research data networks capable of producing high-quality and more reliable data that can support healthcare decisions." By adhering to these standards, organizations can streamline their research processes, reduce the risk of errors, and elevate the overall quality of their trials.
Efficient planning within cross-disciplinary teams is essential for effective risk evaluation in research studies, further underscoring the significance of CCDS medical certification in achieving successful results. At Bioaccess, we offer a comprehensive suite of trial management services, including:
These capabilities not only facilitate quicker patient enrollment—yielding results up to 50% faster—but also lead to substantial cost savings, with $25K saved per patient through our FDA-ready information. This efficiency not only enhances trial outcomes but also positively impacts local economies through job creation and healthcare improvements, fostering international collaboration in the medtech field.
The roots of ccds medical certification stem from the growing necessity for standardized information management practices in research. As clinical trials evolved into intricate global endeavors, the demand for a cohesive method of collecting and reporting information, particularly through ccds medical, became paramount. Regulatory bodies, including the FDA and EMA, have significantly advanced this need by advocating for standardized information formats, which have markedly enhanced the efficiency of the review process. Over time, various organizations and industry associations have actively participated in developing these standards, culminating in the establishment of formal certification processes, such as ccds medical, that ensure compliance with these evolving guidelines. This progression highlights the clinical research community's steadfast dedication to enhancing data quality and ensuring patient safety.
Key milestones in this journey include:
As regulatory bodies continue to influence the development of data standards, the trials environment is poised for ongoing transformation, driven by a commitment to ethical practices and improved patient outcomes.
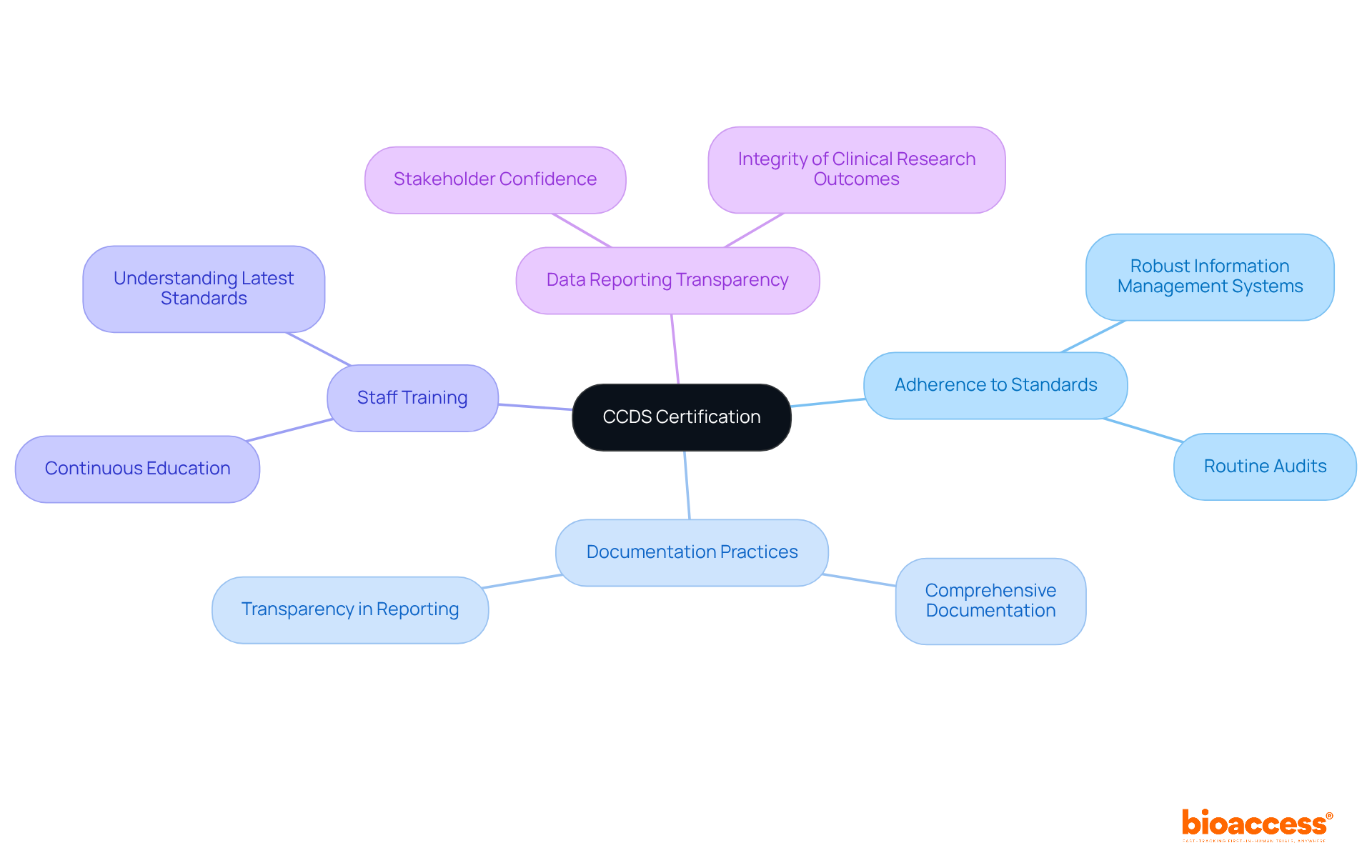
Essential traits of Medical Certification encompass strict adherence to established healthcare information standards, comprehensive documentation practices, and intensive training for staff involved in information management. Organizations pursuing CCDS medical certification must demonstrate their capability to collect and manage medical information in alignment with regulatory guidelines. This necessitates the implementation of robust information management systems, routine audits, and continuous staff training on the latest standards and protocols.
Moreover, transparency in data reporting processes is vital, as it fosters confidence among stakeholders regarding the integrity of clinical research outcomes. Notably, approximately 77% of first-time examinees successfully pass the certification exam, underscoring the importance of thorough preparation and understanding of these requirements.
Furthermore, organizations that prioritize these best practices are more likely to fulfill CCDS medical certification requirements, thereby enhancing their credibility and operational efficiency within the biopharma sector.
CCDS medical certification stands as a cornerstone in clinical research, ensuring organizations adhere to rigorous standards for data management and information integrity. This certification not only elevates research quality but also fosters trust among stakeholders, ultimately leading to improved patient outcomes and a more reliable healthcare system.
The significance of CCDS certification is paramount, as it standardizes clinical data collection and reporting. Key insights reveal its evolution in response to the complexities of global clinical trials, the necessity of compliance with regulatory standards, and the tangible benefits it provides to organizations, including enhanced operational efficiency and improved trial outcomes. Furthermore, the critical characteristics and requirements for achieving this certification underscore a commitment to excellence in research practices.
Reflecting on broader implications, the importance of CCDS certification cannot be overstated. As the healthcare landscape evolves, embracing standardized practices becomes essential for fostering collaboration and innovation across the industry. Organizations are urged to prioritize CCDS certification, not only to enhance their credibility but also as a commitment to advancing the quality of healthcare research. By investing in CCDS certification, stakeholders contribute to a more efficient, transparent, and effective clinical research environment, ultimately benefiting patients and the healthcare community at large.
What is CCDS Medical Certification?
CCDS Medical Certification, or Clinical Standards Medical Certification, is a formal acknowledgment of compliance with established medical research standards, ensuring that medical information is collected, managed, and disclosed according to regulatory standards and industry best practices.
Why is CCDS Medical Certification important for medical trials?
It enhances the reliability of the information produced in medical trials, which is essential for successful regulatory submissions, particularly for Medtech, Biopharma, and Radiopharma firms.
How does CCDS Medical Certification benefit organizations?
By securing this accreditation, organizations optimize their operational efficiency, position themselves favorably in a competitive market, improve patient outcomes, and increase confidence in their innovations.
What services does bioaccess® offer related to trial management?
bioaccess® offers comprehensive trial management services, including feasibility studies, compliance reviews, trial setup, project oversight, and reporting.
What are the costs associated with obtaining CCDS Medical Certification?
The costs vary, with ACDIS members contributing $280 and non-members $380, making it a worthwhile investment for organizations seeking to enhance their operational efficiency and confidence in their innovations.