


This article provides a comprehensive overview of Case Report Forms (CRFs), detailing their definition, evolution, and critical importance in clinical research. It emphasizes the pivotal role CRFs play in data collection and ensuring regulatory compliance.
Particularly, electronic Case Report Forms (eCRFs) are highlighted for their ability to enhance data integrity by significantly reducing errors, improving operational efficiency, and facilitating adherence to stringent regulatory standards. Such advancements are essential for ensuring the reliability of medical research outcomes, thereby reinforcing the trustworthiness of clinical studies.
Understanding the intricacies of Case Report Forms (CRFs) is essential for anyone involved in clinical research. These meticulously crafted documents serve not merely as tools for data collection; they play a pivotal role in ensuring the integrity and compliance of medical studies.
As the landscape of clinical trials evolves—particularly with the shift towards electronic formats—researchers encounter both opportunities and challenges. The design and implementation of CRFs can significantly enhance the quality of research while navigating the complexities of technological advancements.
A Case Report Form (CRF) in research is an essential document in medical studies, meticulously designed to collect information from each participant in a structured manner. It serves as the primary instrument for capturing protocol-required information, ensuring that all relevant details are recorded accurately and consistently. CRFs can be either paper-based or electronic (eCRF), with the latter increasingly favored due to its numerous advantages. Notably, over 80% of Phase III research trials now utilize eCRFs, reflecting a significant shift from traditional paper formats.
The structure of a CRF is dictated by the study protocol, which specifies the information points necessary for evaluating the safety and effectiveness of medical interventions. This organized approach not only facilitates the gathering of critical information but also enhances the overall quality of clinical research. For instance, research indicates that electronic case report forms can reduce entry errors to below 5%, in contrast to manual paper forms, which may exhibit error rates as high as 26%. Furthermore, eCRFs streamline the collection process, with average completion times significantly shorter than their paper counterparts—9.49 minutes for eCRFs compared to 11.32 minutes for paper forms, resulting in a 16% reduction in time.
The significance of CRF in research extends beyond mere data collection; they play a crucial role in ensuring compliance with regulatory standards and maintaining data integrity throughout the research lifecycle. Effective CRF design and implementation can lead to fewer regulatory inquiries during audits, with studies enforcing strict eCRF completion SOPs experiencing up to 30% fewer queries. This underscores the importance of investing in high-quality CRF systems that align with healthcare workflows and regulatory expectations, ultimately supporting the advancement of medical knowledge and improved patient care.
Moreover, bioaccess's comprehensive healthcare study management services, which include feasibility assessments and site selection, enhance the efficacy of CRFs by ensuring that the appropriate information is gathered from the outset. A significant case analysis from 2014 revealed that eCRFs resulted in a reduced total cost per patient and expedited completion of research studies, further underscoring their advantages over conventional paper formats. However, it is also essential to recognize that some investigators have reported technical challenges with eCRFs, highlighting the obstacles that persist in their adoption. As Robert Fleischmann noted, information integrity was superior in the eCRF context, reinforcing the case for their use of CRF in research studies.
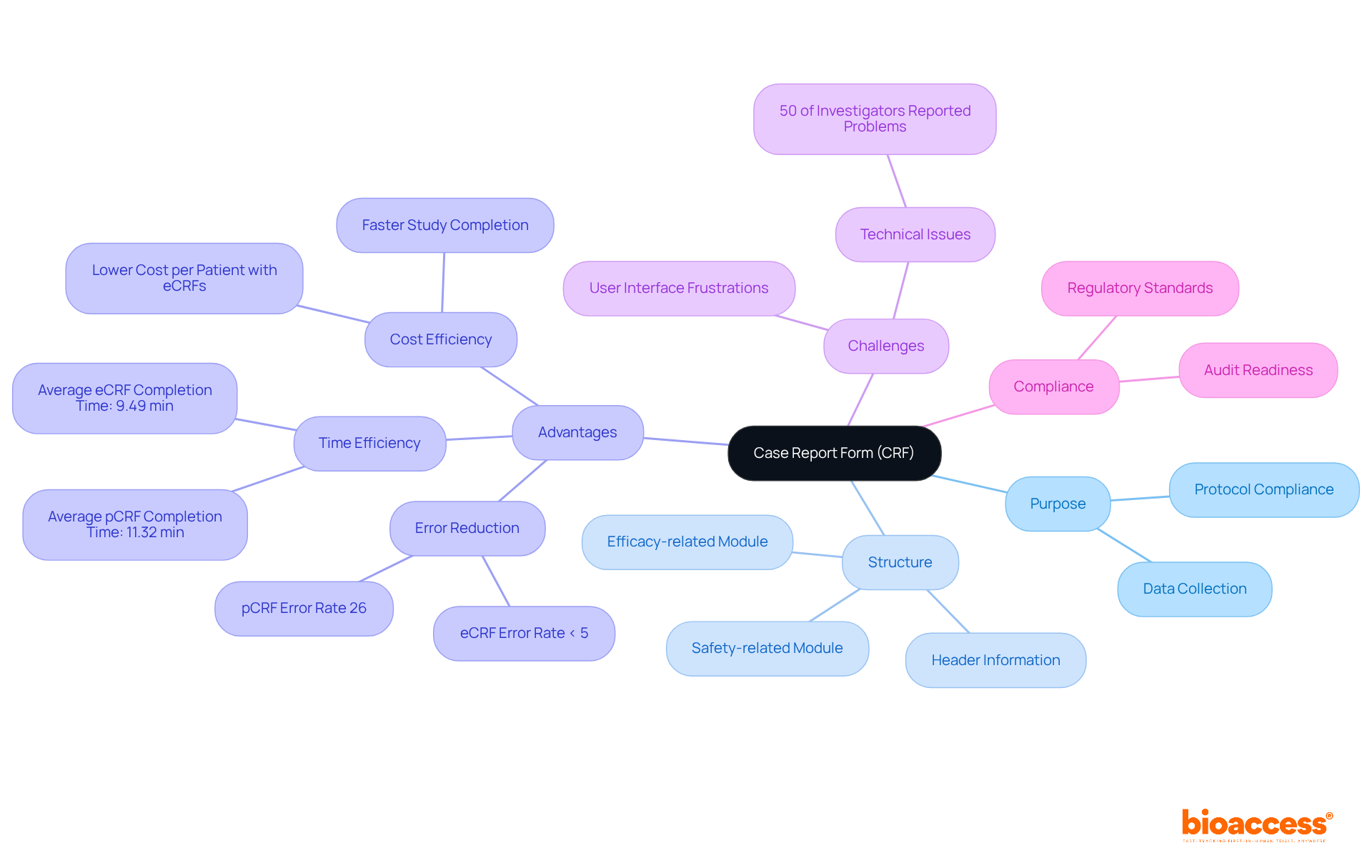
The development of Case Report Forms (CRFs) in research has its roots in the early days of medical research, where information gathering often lacked structure and formality. As clinical trials became more regulated, the demand for standardized information collection tools such as CRFs in research emerged. Initially, CRFs were paper-based, which required meticulous manual entry of data. However, with technological advancements, the introduction of electronic Case Report Forms (eCRFs) has revolutionized the process of information gathering in CRFs in research. eCRFs offer enhanced information management capabilities, including real-time data entry, automated validation checks, and improved accessibility for researchers. This shift reflects a broader trend towards digitization in clinical research, with CRFs in research aimed at boosting efficiency and ensuring the integrity of information. Notably, a 2014 study revealed that 82% of researchers were ready to adopt electronic case report forms in the future, with 31 out of 72 participants preferring electronic formats due to their ease of monitoring and superior quality. While the average time to database lock for electronic case report forms was 31.7 months, compared to 39.8 months for paper forms, it's essential to recognize that this difference was not statistically significant (p = 0.11). Despite the advantages of eCRFs, challenges such as technical issues and the user-unfriendliness of older systems have impeded widespread adoption. Ultimately, this transition underscores the crucial role of technological advancements in shaping the future of clinical research, particularly in enhancing the quality of the information collected related to CRFs in research.

A well-designed CRF in research is critical for clinical research, characterized by clarity, standardization, and adaptability. Key components typically include:
To facilitate straightforward data input and minimize errors, case report forms often incorporate elements like dropdown menus and integrated validation checks. Research indicates that electronic case report forms (eCRFs) significantly reduce entry errors, achieving a 0% error rate compared to a 5% error rate for paper-based forms. Furthermore, patients experience a 23% time savings when utilizing eCRFs versus paper-based CRFs, underscoring their effectiveness.
The use of CRF in research is essential, as standardization across case report forms enhances comparability and regulatory adherence in studies involving human subjects. Additionally, the adaptability of CRFs in research allows for the accommodation of diverse study protocols and patient populations, ensuring they meet specific research needs. This flexibility is essential for enhancing information gathering and elevating the overall quality of medical studies.
As emphasized by the Bioaccess Content Team, adhering to the case report form Completion Guidelines is crucial for improving the reliability and effectiveness of research studies.
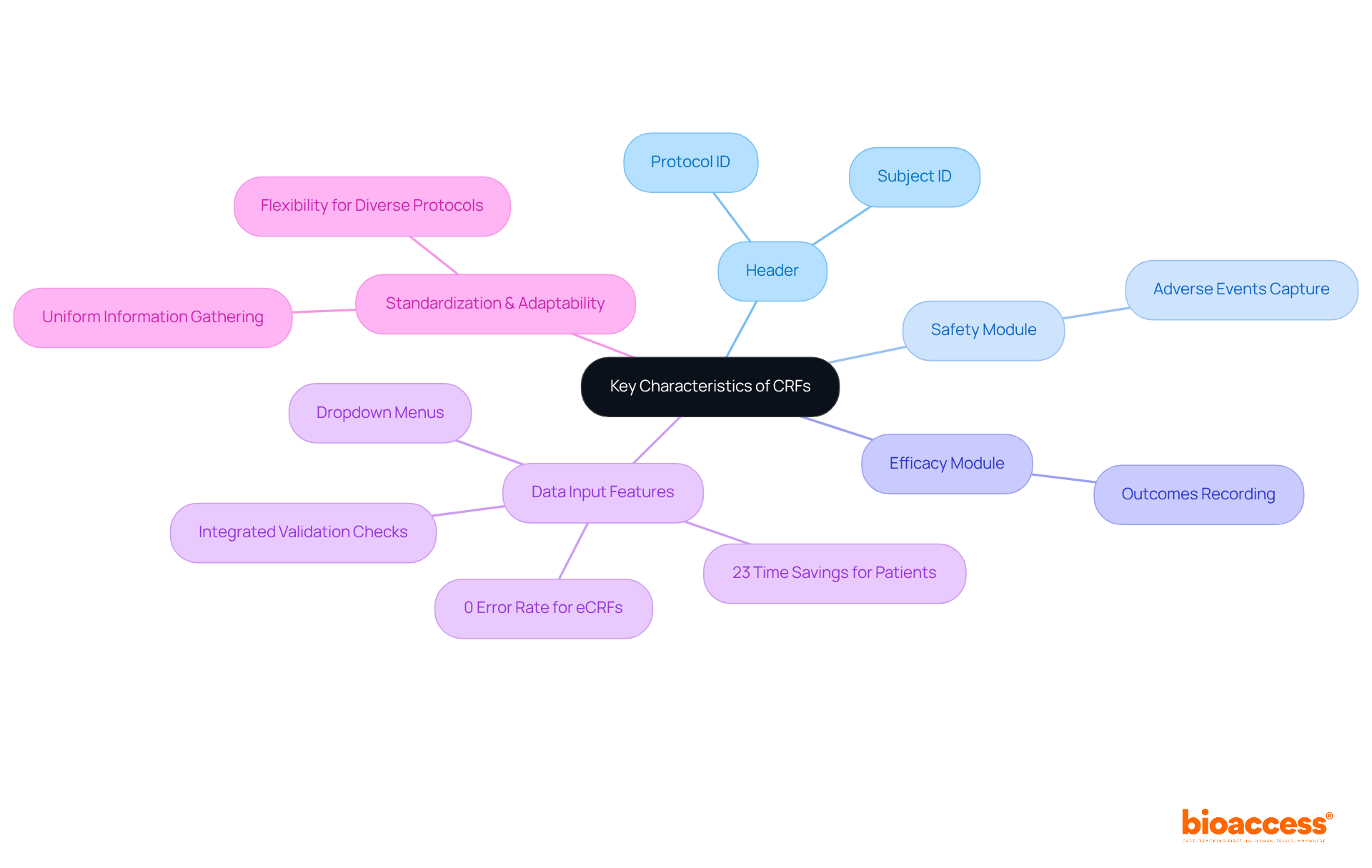
CRFs in research are essential instruments in clinical studies, playing a pivotal role in ensuring information accuracy and compliance with regulatory requirements. By offering an organized format for data collection, CRFs minimize inconsistencies and guarantee that all necessary details are recorded systematically.
Regulatory bodies, including the FDA and EMA, mandate adherence to stringent protocols for data gathering and reporting, which CRFs support by serving as comprehensive records of all trial information. This includes providing an audit trail for modifications, ensuring that data remains intact and accessible for review.
The meticulous design and implementation of CRFs are vital for upholding the integrity of medical research outcomes and safeguarding patient safety. For instance, a study analyzing approximately 15,000 CRFs revealed an overall error rate of just 0.41%, underscoring the effectiveness of well-structured CRFs in maintaining quality.
Furthermore, bioaccess enhances the functionality of research frameworks through its extensive study management services. These services encompass:
Such services streamline data entry processes and significantly reduce the potential for human error, thereby improving compliance rates. As the landscape of medical trials evolves, the significance of CRF in research for ensuring regulatory compliance and protecting participant rights remains paramount.
Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, emphasizes the indispensable role of CRF in research for maintaining high standards of clinical research.

In conclusion, a Case Report Form (CRF) stands as a cornerstone of clinical research, meticulously crafted to gather essential data from participants in a systematic manner. Its significance extends beyond mere data collection; it ensures compliance with regulatory standards and upholds the integrity of the research process. The transition from traditional paper forms to electronic Case Report Forms (eCRFs) marks a pivotal evolution in this field, enhancing both efficiency and accuracy while addressing the challenges of data management.
Key points throughout this discussion highlight the advantages of eCRFs, including reduced error rates and faster completion times, which contribute to improved data quality. The historical context reveals how CRFs have adapted over time to meet the increasing demands of medical research, evolving from basic paper formats to sophisticated electronic systems. The necessity for clarity, standardization, and adaptability in CRF design is paramount, showcasing their role in facilitating compliance and maintaining the integrity of clinical trials.
The importance of CRFs in research cannot be overstated; they are integral to ensuring the reliability of medical studies and protecting participant rights. As the landscape of clinical research continues to evolve, embracing technological advancements and adhering to best practices in CRF development will be crucial. Investing in high-quality CRF systems not only enhances data integrity but also supports the advancement of medical knowledge, ultimately leading to improved patient care. Engaging with these tools and practices is essential for researchers aiming to navigate the complexities of modern clinical trials effectively.
What is a Case Report Form (CRF)?
A Case Report Form (CRF) is an essential document in clinical research designed to collect structured information from each participant in a medical study.
What is the purpose of a CRF in clinical research?
The purpose of a CRF is to capture protocol-required information accurately and consistently, facilitating the evaluation of the safety and effectiveness of medical interventions.
What types of CRFs are used in research?
CRFs can be either paper-based or electronic (eCRF), with electronic formats increasingly favored due to their advantages.
What are the advantages of using electronic CRFs (eCRFs)?
eCRFs reduce entry errors to below 5%, streamline the collection process, and have shorter average completion times compared to paper forms, resulting in a 16% reduction in time.
How does the structure of a CRF relate to the study protocol?
The structure of a CRF is dictated by the study protocol, which specifies the necessary information points for evaluating medical interventions.
How do CRFs ensure compliance with regulatory standards?
CRFs play a crucial role in ensuring compliance with regulatory standards and maintaining data integrity throughout the research lifecycle, leading to fewer regulatory inquiries during audits.
What impact does effective CRF design have on research studies?
Effective CRF design and implementation can lead to fewer regulatory inquiries, with studies enforcing strict eCRF completion SOPs experiencing up to 30% fewer queries.
How do CRFs contribute to the advancement of medical knowledge?
By supporting the collection of high-quality data that aligns with healthcare workflows and regulatory expectations, CRFs ultimately contribute to improved patient care and the advancement of medical knowledge.
What challenges are associated with the adoption of eCRFs?
Some investigators have reported technical challenges with eCRFs, indicating obstacles that persist in their adoption despite their advantages.
What conclusions can be drawn from case analyses regarding eCRFs?
Case analyses have shown that eCRFs can lead to reduced total costs per patient and expedited completion of research studies compared to conventional paper formats.