


The FDA classification of medical devices is of paramount importance, as it categorizes products into three distinct classes based on their risk levels. This classification directly influences compliance requirements and the approval processes necessary for market access. Understanding these classifications is vital for manufacturers; it significantly affects the timeline for bringing devices to market and the extent of clinical research required. Notably, Class III devices encounter the most stringent regulations and a longer approval process compared to their Class I and II counterparts.
Understanding the FDA classification of medical devices is crucial for manufacturers navigating the complex landscape of medical regulations. This classification system not only dictates the level of oversight required but also influences the speed at which devices can enter the market. The implications are significant, impacting everything from compliance measures to clinical research strategies. With the growing number of recalls, particularly among moderate-risk devices, the stakes are higher than ever. Manufacturers must consider:
The process of medical equipment classification is essential, particularly the FDA classification of medical devices, which organizes items based on their intended use, associated risk levels, and compliance requirements. The FDA classification of medical devices categorizes instruments into three primary groups: Group I, Group II, and Group III. Each class is governed by distinct oversight measures and criteria, which are essential for ensuring the safety and efficacy of these instruments.
Category I products, representing approximately 35% of the medical equipment sector, pose the least risk and are subject to minimal regulatory oversight, often exempt from premarket notification (510(k)). Conversely, Category II products, accounting for around 53% of authorized items, require a 510(k) submission to demonstrate substantial equivalence to existing products, along with adherence to additional special controls tailored to their specific risks. Category III products, constituting about 9% of the industry, face the most stringent standards, including the Premarket Approval (PMA) process, which demands extensive scientific data to ensure safety and efficacy.
Understanding the FDA classification of medical devices is vital for manufacturers, as it dictates the necessary compliance measures and the types of premarket submissions that are required. For instance, a Class III product may require comprehensive clinical trials and post-launch monitoring, significantly influencing the timeline and success of bringing a medical item to market. Recent trends indicate a growing number of recalls, particularly among Category II devices, underscoring the importance of rigorous quality control and adherence to FDA standards.
Real-world examples illustrate the impact of classification on market entry. For example, a client’s in vitro diagnostic (IVD) product was classified as Class III in Japan, necessitating local clinical trials due to the absence of comparable products. This complexity emphasizes the need for manufacturers to adeptly navigate regulatory environments to ensure compliance and expedite their market entry. In summary, the FDA classification of medical devices not only influences compliance but also plays a crucial role in determining the speed at which medical instruments can reach patients, making it a vital step in the product development process.
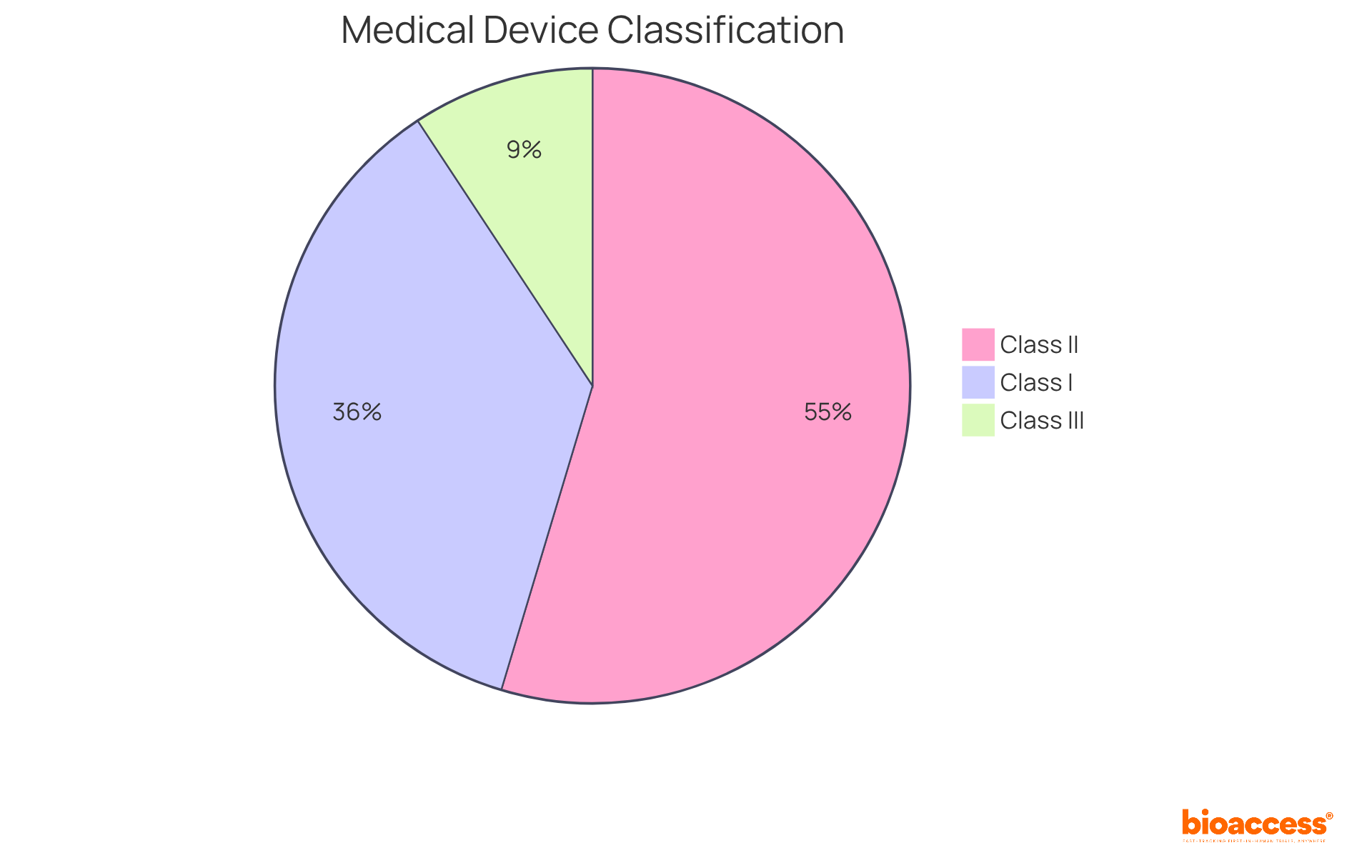
The FDA classification of medical devices includes three distinct categories based on their risk levels and regulatory requirements.
Class I: These devices are categorized as low-risk and are subject to minimal regulatory oversight. Common examples include bandages, tongue depressors, and manual stethoscopes. Notably, the majority of Class I items are exempt from premarket notification obligations, which facilitates their introduction into the market.
Class II: Devices in this category pose moderate risk and require stricter regulatory controls. Typically, they necessitate a 510(k) premarket notification to demonstrate substantial equivalence to an already marketed product. Examples of Class II devices include powered wheelchairs, infusion pumps, and surgical drapes. The Class II market is expected to expand considerably, reflecting the rising demand for these essential medical technologies.
Class III: These high-risk instruments are subject to the most stringent regulatory controls, including premarket approval (PMA). Class III devices are critical for aiding or maintaining human existence, are frequently implanted, or present a significant risk of disease or harm. Examples include pacemakers, breast implants, and defibrillators. The rigorous requirements for Class III products are designed to ensure their safety and efficacy before they enter the market.
Understanding the FDA classification of medical devices is vital for compliance and for effectively navigating the regulatory landscape. As the medical equipment industry continues to evolve, staying informed about these categories and their implications will be essential for innovators and stakeholders alike.
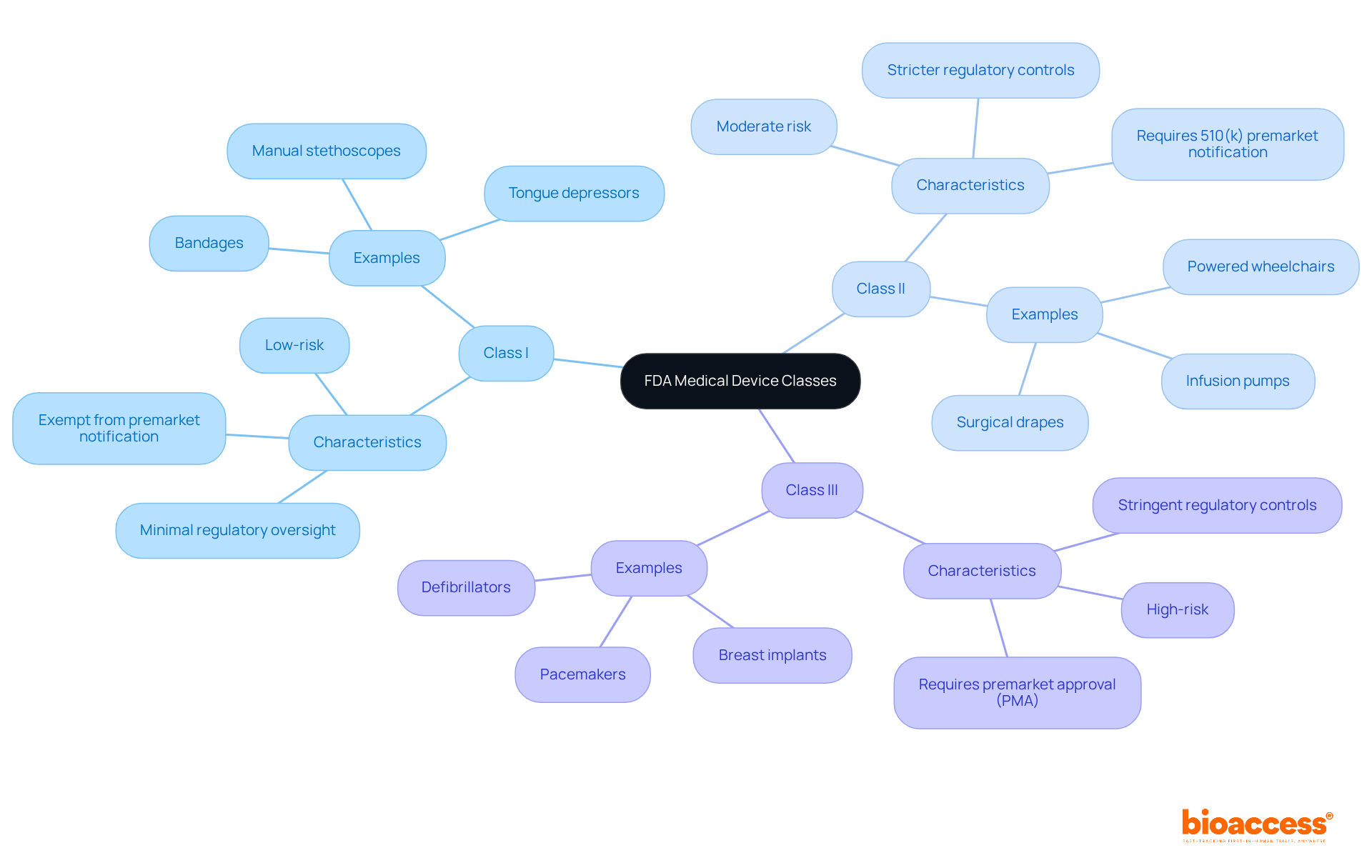
Understanding the FDA classification of medical devices is crucial for manufacturers, as the approval processes vary considerably across device classes in the regulatory landscape.
Class I devices generally enjoy exemptions from premarket notification. However, manufacturers must still register their establishments and list their devices with the FDA. Adherence to general controls is vital to guarantee safety and effectiveness.
Class II devices necessitate a 510(k) premarket notification, requiring manufacturers to demonstrate substantial equivalence to an existing product. The average review time for 510(k) submissions is around 90 days, although this period can extend if the FDA requests additional information. Notably, in the year leading up to September 2022, 67 percent of 510(k) submissions resulted in requests for further information, underscoring the necessity for meticulous documentation and timely responses during the review process.
Class III products face the most stringent approval process, necessitating a Premarket Approval (PMA) application. This pathway demands extensive clinical trials to establish safety and efficacy, often spanning several months to years. The PMA process entails a thorough examination of the product's design, manufacturing practices, and labeling, with decisions typically issued within 180 days of submission. However, this timeframe can be misleading due to potential deficiencies in the application.
Grasping the FDA classification of medical devices is essential for manufacturers to effectively navigate the complexities of approval.
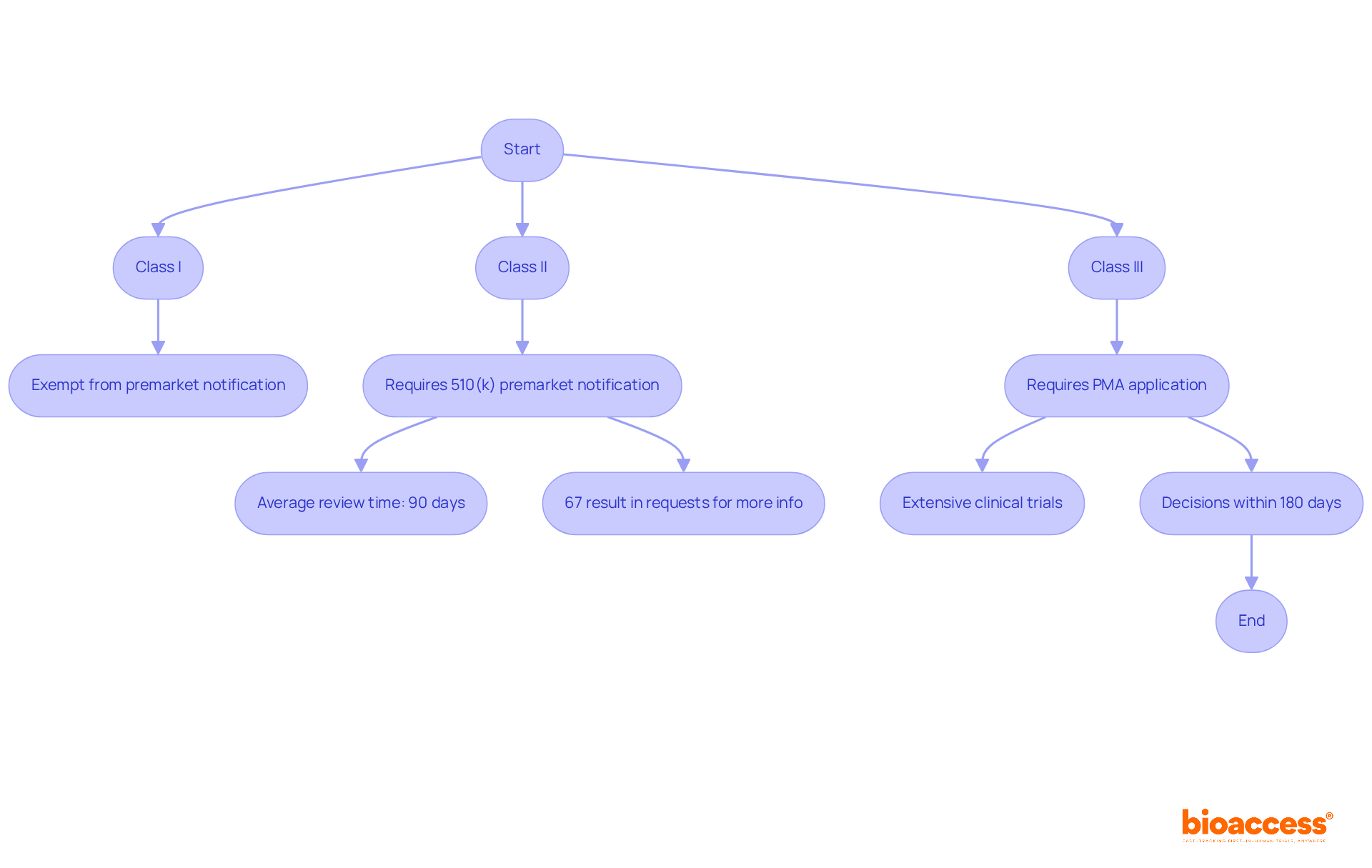
The FDA classification of medical devices is pivotal in shaping both clinical research and market access strategies. Understanding the FDA classification of medical devices is essential for navigating the complexities of the Medtech landscape.
Clinical Research: The classification directly influences the type and extent of clinical trials required. Category III products, which are high-risk and frequently life-sustaining, typically necessitate comprehensive clinical trials to demonstrate safety and effectiveness. In contrast, Category I products, regarded as low-risk, may not require any clinical data, significantly shortening the timeline and resources needed for research. This disparity can lead to extended development phases for Category III items, ultimately affecting the FDA classification of medical devices and their overall time to market.
The compliance pathway and speed of entry into the industry are influenced by the FDA classification of medical devices. Category I products generally enjoy a quicker introduction due to fewer compliance hurdles, often being exempt from premarket notification (510(k)). Conversely, Category III products face stringent requirements, such as the premarket approval (PMA) process, which can delay access to the market. For example, the average FDA decision time for a 510(k) application is approximately five months, while the PMA process can take significantly longer, often requiring extensive documentation and clinical studies. Furthermore, understanding the nuances of the FDA classification of medical devices enables manufacturers to align their promotional strategies with compliance expectations, facilitating smoother entry into the industry.
As we look ahead to 2025, access challenges for Class III devices remain pronounced. Manufacturers must navigate complex compliance landscapes and potential misclassification risks that could result in substantial fines or product recalls. Expert insights emphasize the importance of early engagement with oversight organizations to streamline the approval process and mitigate these challenges. By leveraging strategic partnerships and regulatory expertise, companies can bolster their chances of successful market access, particularly in competitive environments.
The FDA classification of medical devices stands as a cornerstone of regulatory compliance, categorizing devices based on their intended use and associated risk levels. This classification system not only dictates the regulatory requirements that manufacturers must adhere to but also significantly impacts the speed at which medical devices can be brought to market. Understanding these classifications is crucial for manufacturers aiming to navigate the complex landscape of medical device approval effectively.
Key points highlighted throughout the article include the three primary classes of medical devices—Class I, Class II, and Class III—each with distinct regulatory frameworks:
The implications of these classifications extend beyond compliance; they influence clinical research strategies, market access timelines, and the overall feasibility of introducing new medical technologies.
As the medical device industry continues to evolve, staying informed about the FDA classification system is essential. Manufacturers are encouraged to engage proactively with regulatory bodies and to ensure a thorough understanding and adherence to compliance requirements. By doing so, they can mitigate risks associated with misclassification and potential recalls while enhancing their ability to deliver innovative medical solutions to patients in a timely manner. Emphasizing the importance of this knowledge empowers stakeholders to navigate the challenges of the regulatory landscape effectively, ultimately improving patient outcomes and advancing healthcare technology.
What is medical device classification?
Medical device classification is the process of organizing medical equipment based on its intended use, associated risk levels, and compliance requirements.
Why is FDA classification of medical devices important?
The FDA classification is important because it dictates the necessary compliance measures and types of premarket submissions required for medical devices, ensuring their safety and efficacy.
How are medical devices categorized by the FDA?
The FDA categorizes medical devices into three primary groups: Group I (Class I), Group II (Class II), and Group III (Class III), each with distinct oversight measures and criteria.
What are the characteristics of Class I medical devices?
Class I devices pose the least risk, represent about 35% of the medical equipment sector, and are subject to minimal regulatory oversight, often exempt from premarket notification (510(k)).
What are the requirements for Class II medical devices?
Class II devices require a 510(k) submission to demonstrate substantial equivalence to existing products and must adhere to additional special controls tailored to their specific risks, accounting for around 53% of authorized items.
What distinguishes Class III medical devices from the other classes?
Class III devices face the most stringent standards, constituting about 9% of the industry, and require the Premarket Approval (PMA) process, which demands extensive scientific data to ensure safety and efficacy.
How does the classification impact the market entry of medical devices?
The classification influences the compliance measures, types of premarket submissions needed, and can significantly affect the timeline and success of bringing a medical device to market.
What recent trends have been observed in the recall of medical devices?
There has been a growing number of recalls, particularly among Class II devices, highlighting the importance of rigorous quality control and adherence to FDA standards.
Can you provide an example of how classification affects regulatory requirements?
An example is a client’s in vitro diagnostic (IVD) product classified as Class III in Japan, which required local clinical trials due to the absence of comparable products, illustrating the complexity of navigating regulatory environments.