


Understanding the complexities of Good Clinical Practice (GCP) is crucial for anyone involved in clinical research, especially in Bulgaria, where adherence to both EU regulations and local laws is vital. This article explores the significance of GCP deviations and the reporting processes that uphold the integrity of clinical trials.
But what occurs when these deviations arise? How can researchers effectively navigate the challenges of compliance while ensuring participant safety and data integrity? Addressing these questions underscores the essential role that strict adherence to GCP principles plays in the success of medical research.
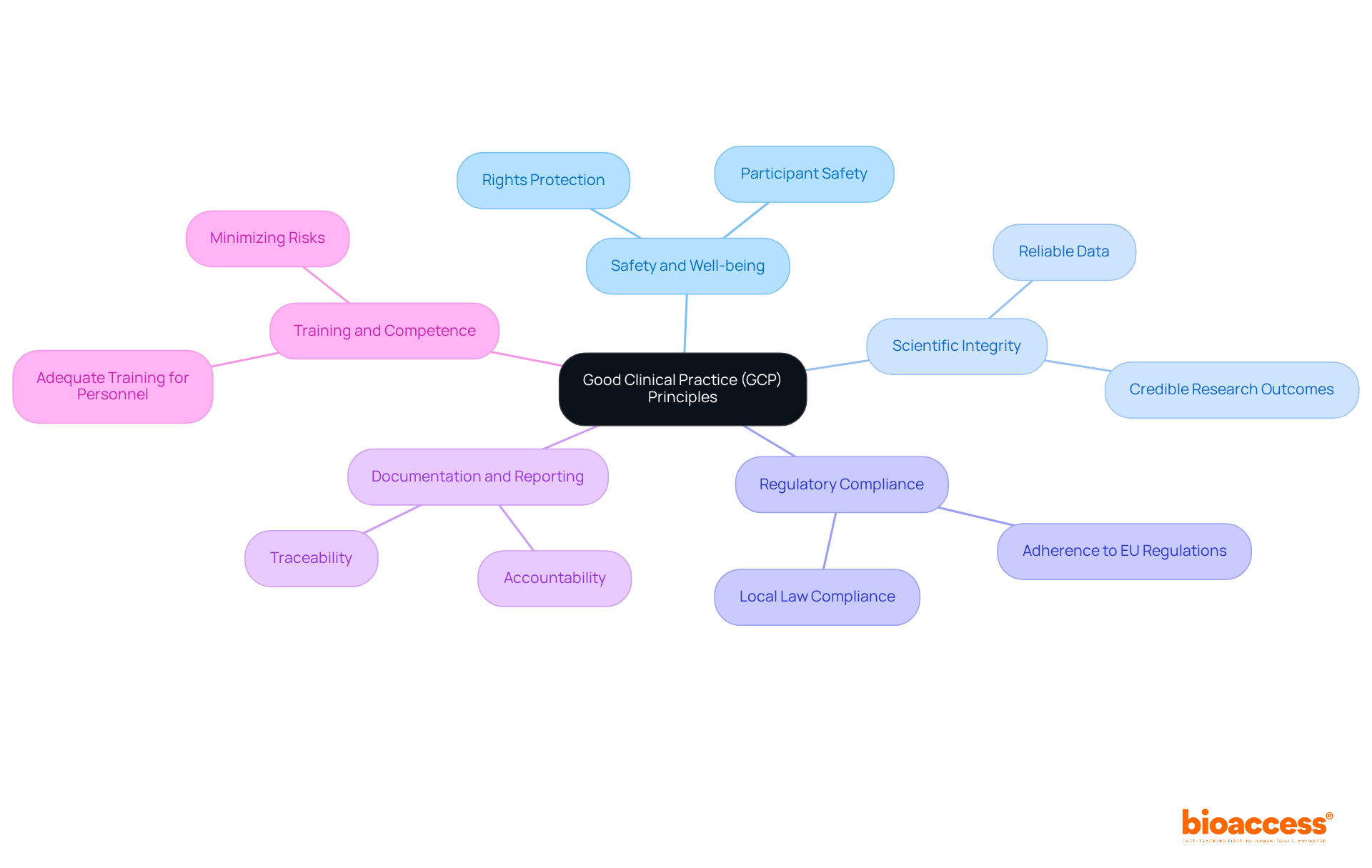
Good Clinical Practice (GCP) represents a comprehensive framework of globally recognized ethical and scientific quality standards that are vital for the design, execution, documentation, and reporting of studies involving human subjects. Understanding GCP deviations and reporting in Bulgaria is essential for maintaining the integrity and success of medical research, particularly because compliance with both EU regulations and local laws is mandatory. The fundamental principles of GCP include:
At Bioaccess, we offer comprehensive management services for studies that directly uphold these GCP principles. Our services include feasibility studies to assess site suitability, site selection to ensure optimal participant recruitment, compliance reviews to meet regulatory standards, study setup for efficient execution, import permits for investigational devices, project management for oversight, and thorough reporting to maintain transparency. Understanding and applying these principles is crucial for researchers, sponsors, and regulatory bodies to ensure that studies are conducted ethically and effectively.
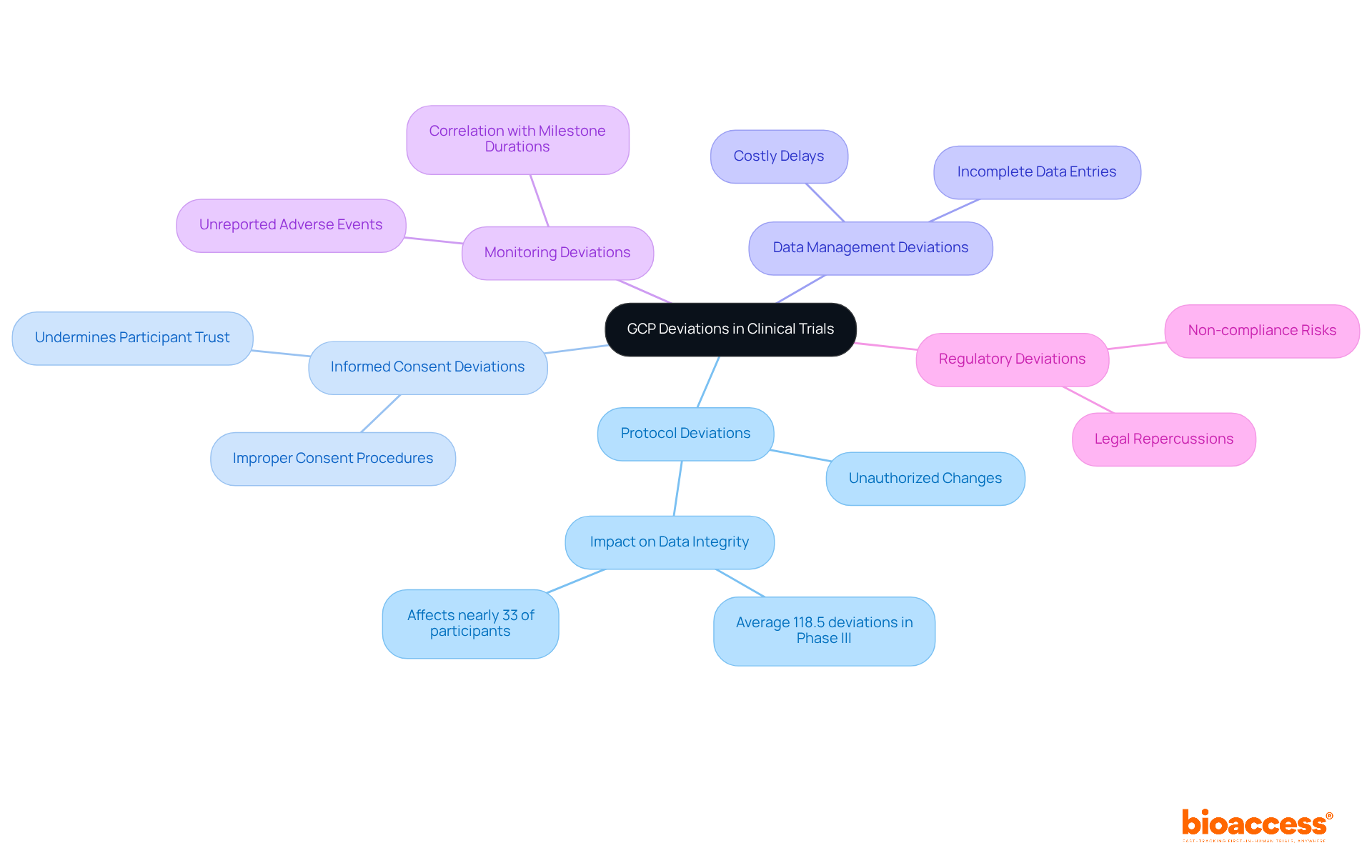
GCP deviations can be classified into several categories, each carrying distinct implications for clinical trials:
Protocol Deviations: These unauthorized changes from the approved study protocol, such as enrolling ineligible participants or missing scheduled visits, are significant. A typical Phase III study experiences roughly 118.5 deviations, impacting nearly 33% of participants. This statistic emphasizes the essential need for adherence to protocols to uphold data integrity.
Informed Consent Deviations: These occur when informed consent is not properly obtained. This may involve using outdated consent forms or failing to re-consent after protocol amendments. Such lapses can undermine participant trust and the ethical foundation of the study.
Data Management Deviations: Issues in data collection and management, including incomplete or flawed data entries, can significantly affect study outcomes. Efficient data management is crucial; discrepancies in this area can lead to costly delays and regulatory scrutiny.
Monitoring Deviations: Failures in monitoring processes can result in unreported adverse events or insufficient oversight of study conduct. Ongoing observation is vital, as research indicates that the occurrence of protocol discrepancies correlates positively with milestone durations. This highlights the significance of robust monitoring practices.
Regulatory Deviations: Non-compliance with local regulatory requirements can lead to severe legal repercussions and jeopardize the study's validity. Regulatory authorities meticulously examine how anomalies are monitored and documented, making it crucial for sponsors to maintain strict compliance.
Identifying and addressing these discrepancies is essential for preserving the integrity of research studies and ensuring participant safety. As the landscape of medical research evolves, particularly with the integration of new technologies and methodologies, proactive oversight of GCP discrepancies will become increasingly vital.
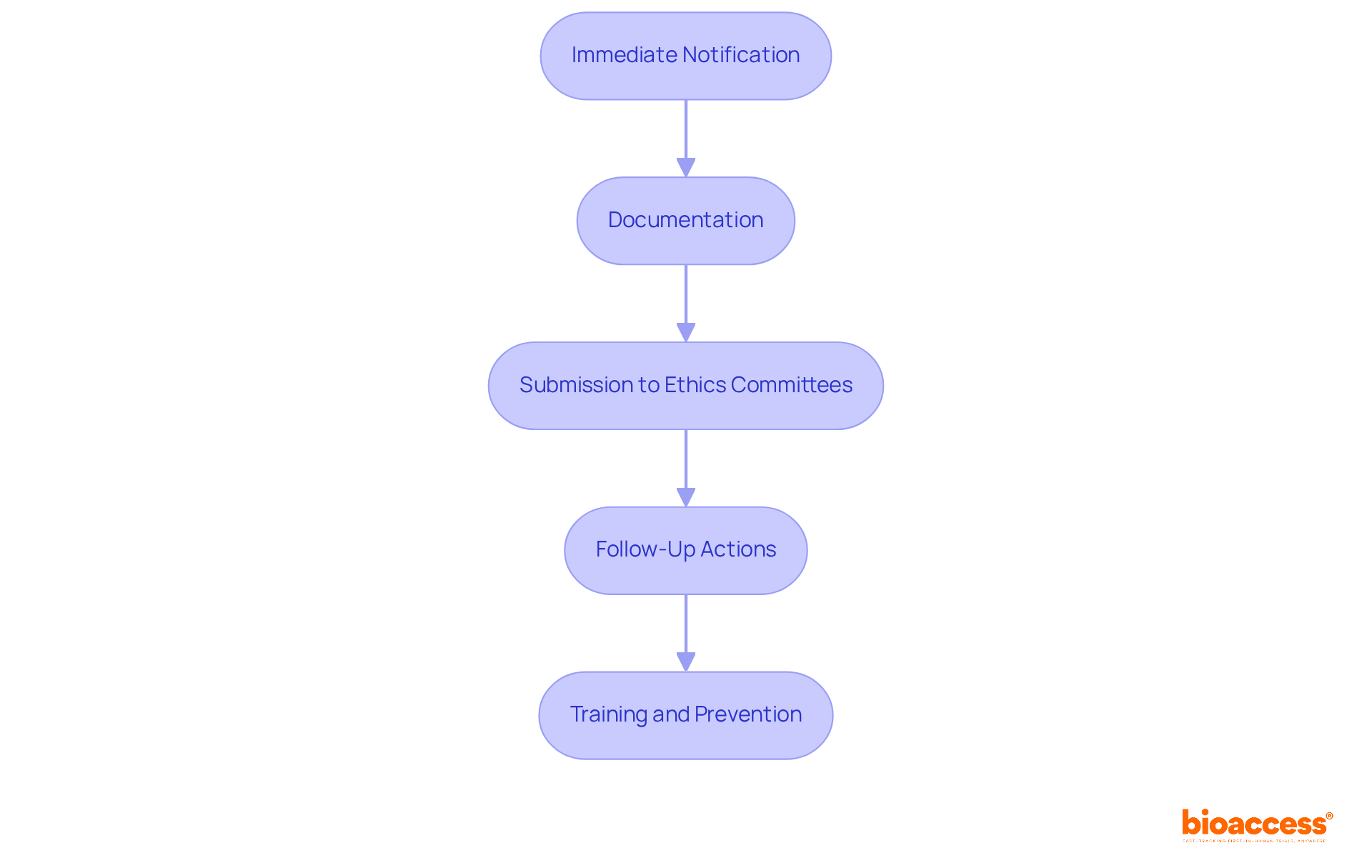
The reporting process for GCP deviations is crucial for upholding the integrity of clinical trials, encompassing several key steps:
Immediate Notification: Investigators must promptly inform the sponsor and relevant regulatory authorities upon identifying a discrepancy, especially if it poses a risk to participant safety. Timeliness is vital; variations impacting primary endpoints or subject rights should be reported within 24-72 hours.
Documentation: A comprehensive report must be prepared, detailing the nature of the discrepancy, its impact on the study, and any corrective actions taken. This report should include:
Submission to Ethics Committees: Depending on the seriousness of the divergence, the report may need to be submitted to the Institutional Review Board (IRB) or ethics committee for review. This step is vital for maintaining ethical standards and participant safety.
Follow-Up Actions: Implement necessary corrective measures and monitor for the recurrence of similar discrepancies. Efficient handling of variances is crucial to avoid escalation into significant compliance risks.
Training and Prevention: Conduct regular training sessions for staff to reduce the likelihood of future discrepancies and ensure adherence to GCP. Ongoing, scenario-based training reinforces key instructions and reduces errors due to misinterpretation or procedural drift.
By adhering to this organized reporting procedure, research teams can enhance accountability and uphold the highest ethical standards in their studies.
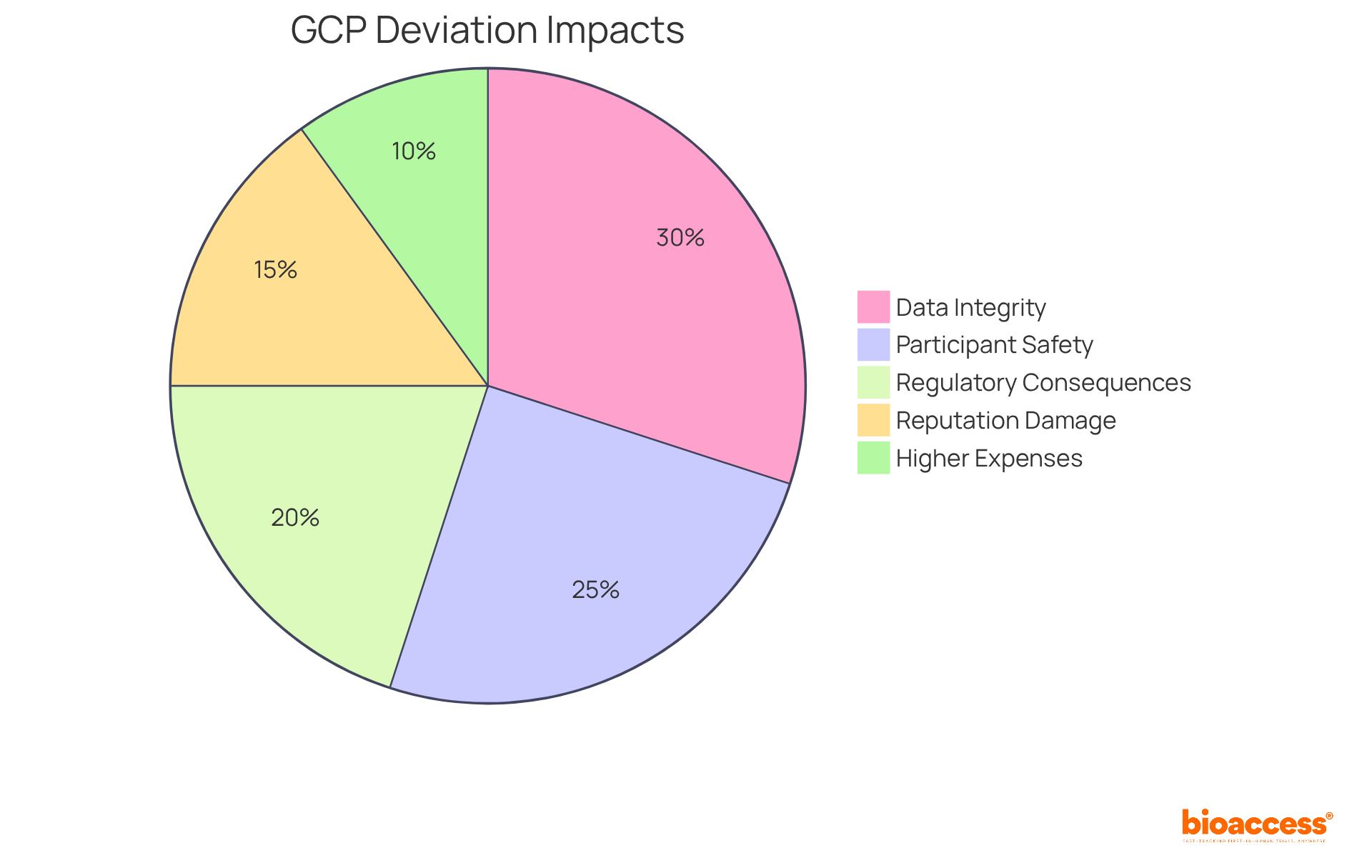
GCP deviations can have profound implications for clinical trials, particularly in the following areas:
Data Integrity: Deviations can significantly compromise the quality and reliability of collected data, potentially leading to invalid study results. Even slight variations, like missed evaluations or incomplete records, can introduce bias and impact statistical power, ultimately compromising the validity of the study.
Participant Safety: Non-compliance with GCP standards can heighten risks to participants, jeopardizing their safety and well-being. For instance, enrolling ineligible subjects or failing to obtain proper informed consent exposes participants to unforeseen risks, which can have serious ethical and legal ramifications.
Regulatory Consequences: Regulatory authorities may impose severe penalties, including fines or suspension of the trial, if discrepancies are not adequately addressed. The FDA, for example, may issue warning letters for frequent non-compliance, which can lead to disqualification of clinical investigators and hinder future research opportunities.
Reputation Damage: Frequent or severe divergences can tarnish the reputation of the sponsoring organization and the investigators involved, leading to a loss of trust from stakeholders. This erosion of confidence can have long-lasting effects on future collaborations and funding opportunities.
Higher Expenses: Tackling discrepancies frequently requires extra resources, such as time and funding, which can raise the total expense of the study. The financial pressures for quicker recruitment and study completion can unintentionally raise the chances of protocol violations, establishing a difficult cycle for sponsors.
Understanding these implications highlights the critical need for strict adherence to GCP principles, particularly in relation to GCP deviations and reporting in Bulgaria, to ensure successful trial outcomes. Recent case studies have shown that robust quality assurance systems and ongoing training for site staff are essential in mitigating risks associated with GCP deviations and reporting in Bulgaria, ultimately safeguarding participant safety and data integrity.
Understanding GCP deviations and the reporting process in Bulgaria is crucial for the integrity and success of clinical trials. Adhering to Good Clinical Practice not only ensures the safety and well-being of participants but also upholds the scientific integrity of research. By recognizing the importance of compliance with both EU regulations and local laws, researchers and sponsors can significantly enhance the quality of their studies.
This article has highlighted various types of GCP deviations, including:
Each of these deviations poses distinct risks to participant safety, data integrity, and the overall credibility of clinical trials. Furthermore, the outlined reporting process emphasizes the necessity of timely notification, thorough documentation, and proactive corrective actions to mitigate the consequences of these deviations.
In conclusion, the implications of GCP deviations extend beyond immediate study outcomes; they can affect participant trust, regulatory relationships, and the reputation of research organizations. It is imperative for all stakeholders involved in clinical trials to prioritize adherence to GCP principles and establish robust quality assurance systems. By doing so, the potential risks associated with deviations can be minimized, ultimately leading to more reliable and ethical research outcomes. Engaging in continuous training and maintaining open communication are vital steps toward fostering a culture of compliance that benefits everyone involved in the clinical research process.
What is Good Clinical Practice (GCP)?
Good Clinical Practice (GCP) is a comprehensive framework of globally recognized ethical and scientific quality standards essential for the design, execution, documentation, and reporting of studies involving human subjects.
Why is understanding GCP deviations and reporting important in Bulgaria?
Understanding GCP deviations and reporting in Bulgaria is crucial for maintaining the integrity and success of medical research, as compliance with both EU regulations and local laws is mandatory.
What are the fundamental principles of GCP?
The fundamental principles of GCP include the safety and well-being of study participants, scientific integrity of the research, regulatory compliance, clear documentation and reporting practices, and training and competence of personnel involved in research studies.
What is the priority in GCP concerning study participants?
The foremost priority in GCP is the rights, safety, and well-being of study participants, ensuring their interests are protected throughout the research process.
How does GCP ensure the reliability of research outcomes?
GCP mandates that the information generated from medical studies must be reliable and credible, fostering confidence in the research outcomes.
What role does regulatory compliance play in GCP?
Regulatory compliance is crucial in GCP as trials must conform to relevant regulatory requirements to uphold the ethical standards of research involving human subjects.
Why is documentation important in GCP?
Clear documentation and reporting practices are essential for accountability and traceability in GCP deviations and reporting.
What training is required for personnel involved in research studies?
All personnel involved in research studies must receive adequate training in GCP principles to minimize risks and ensure high-quality research.
What services does Bioaccess offer to uphold GCP principles?
Bioaccess offers comprehensive management services including feasibility studies, site selection, compliance reviews, study setup, import permits for investigational devices, project management, and thorough reporting.
Who benefits from understanding and applying GCP principles?
Understanding and applying GCP principles is crucial for researchers, sponsors, and regulatory bodies to ensure that studies are conducted ethically and effectively.