


The article underscores the pivotal role of Informed Consent Forms (ICFs) in clinical research, asserting their significance for ethical participation, legal compliance, and the cultivation of trust between researchers and participants. It elaborates on the essential components of effective ICFs while addressing challenges such as complex language and cultural differences that impede participant understanding. This ultimately emphasizes the urgent need for clarity and ongoing communication throughout the consent process.
Informed Consent Forms (ICFs) are the cornerstone of ethical practices in clinical research. Yet, many participants struggle to grasp their full implications. These documents not only outline the purpose, risks, and rights associated with a study but also embody a commitment to transparency and participant autonomy.
The challenge remains: how can researchers ensure that consent forms are not only legally compliant but also easily understood by diverse populations?
Exploring the intricacies of ICFs reveals their significance in safeguarding participant rights and underscores the pressing need for innovation in communication to bridge the comprehension gap.
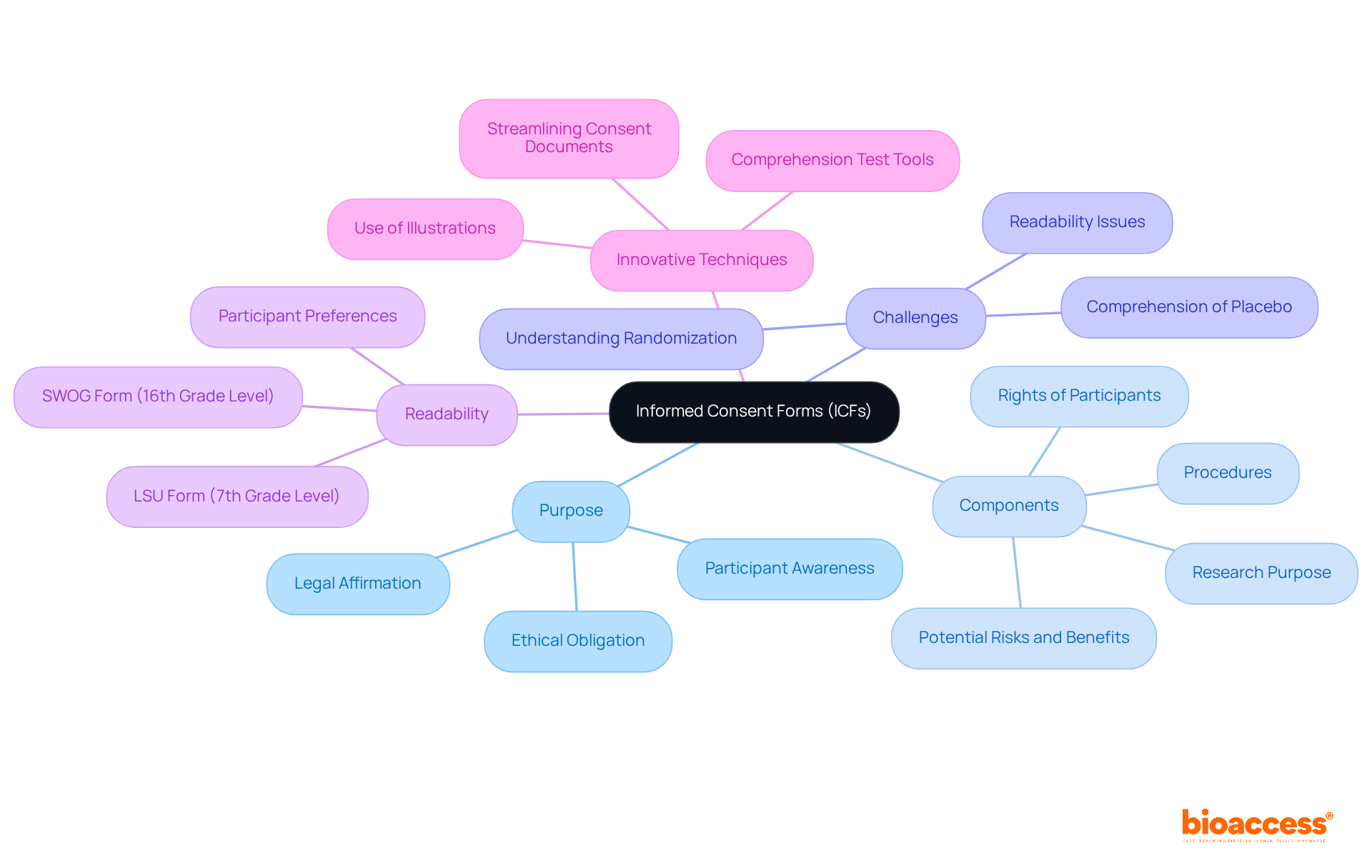
Informed Consent Forms (ICFs) are vital documents that highlight the importance of ICF in clinical research, ensuring individuals are fully aware of the studies they are considering. The ICF in clinical research delineates the research's purpose, procedures, potential risks and benefits, and the rights of participants. This document acts as a legal affirmation of the individual's voluntary consent to participate in the study after receiving adequate information.
It is imperative that the ICF in clinical research is clear, concise, and tailored to the specific study context, enabling individuals to comprehend all relevant details prior to granting consent. This process transcends mere formality; it represents a fundamental ethical obligation that upholds the autonomy and rights of individuals involved in research.
Notably, research indicates that while approximately 75% of clinical trial volunteers understand various aspects of informed consent, comprehension of complex elements such as randomization and placebo concepts has stagnated over the past thirty years. Therefore, streamlining consent documents and employing innovative communication methods are recommended to enhance understanding among participants and ensure ethical standards are met.
As Naveen Dha asserts, 'Various innovative communication techniques are now being recommended to improve communication and enhance the agreement process to tackle these issues.' Furthermore, the SWOG agreement form was assessed at a 16th-grade reading level, whereas the LSU form was at a 7th-grade reading level, underscoring the readability challenges faced by participants. In fact, 62% of individuals preferred the LSU form over the SWOG form, highlighting the critical need for simplifying consent documentation.
The significance of Informed Consent Forms (ICFs) in clinical research is paramount, serving multiple essential functions:
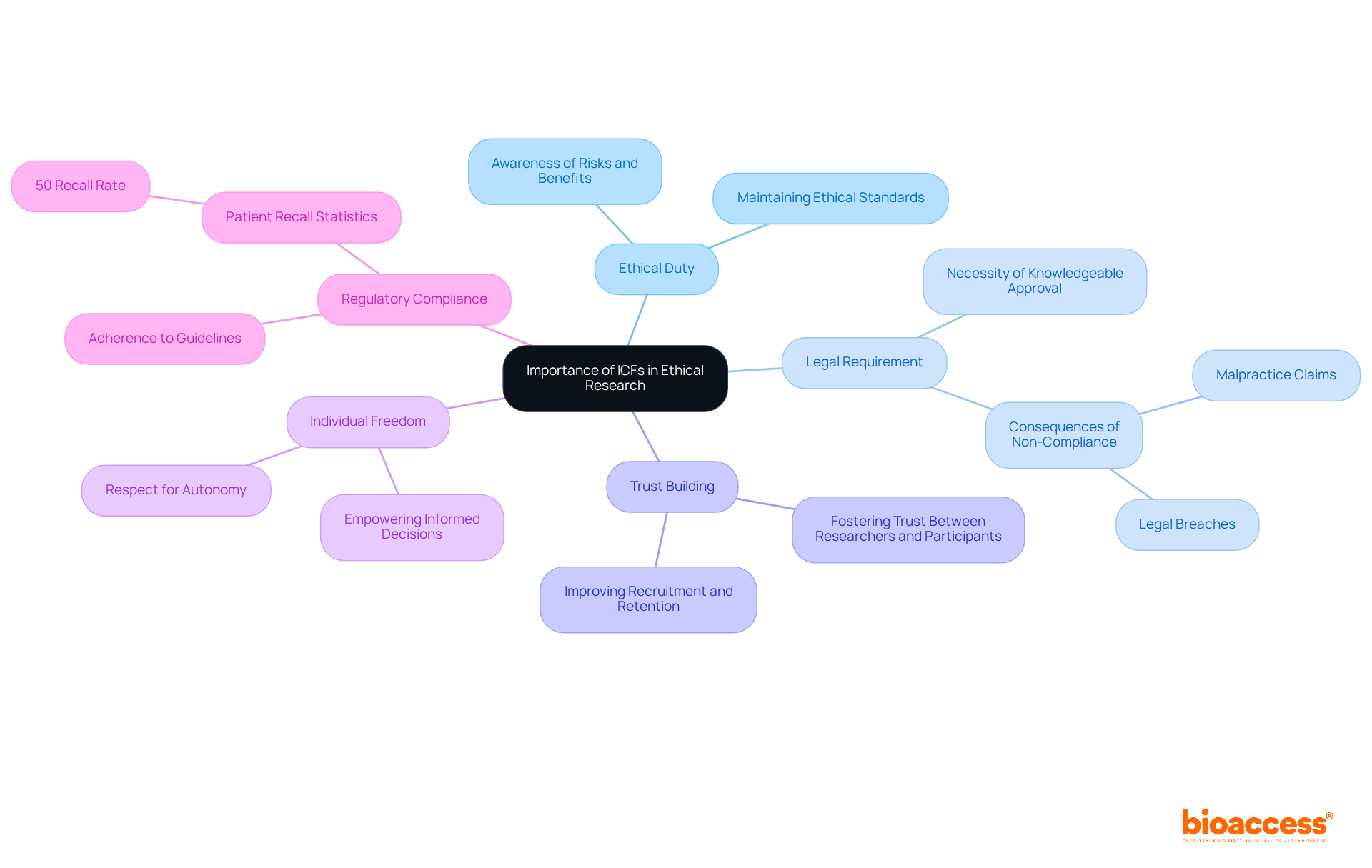
Ethical Duty: ICFs are essential to ethical study practices, guaranteeing individuals are completely aware of their involvement, including related risks and benefits. This clarity is essential for maintaining ethical standards in studies.
Legal Requirement: According to the law, acquiring knowledgeable approval is necessary for including participants in clinical trials. Non-compliance can result in severe legal repercussions, including malpractice claims and ethical breaches, jeopardizing the integrity of the research. A situation in which a doctor performed surgery on the incorrect ear underscores the legal consequences of not obtaining adequate consent.
Trust Building: Comprehensive ICFs foster trust between researchers and participants. When individuals feel sufficiently knowledgeable and valued, they are more likely to engage in the study process, improving recruitment and retention. Almost 50% of studies address ethical approval and consent, highlighting the frequency of ICF discussions in clinical studies.
Individual Freedom: ICFs empower individuals by allowing them to make educated decisions about their engagement in studies. This respect for autonomy is a core principle of ethical research, reinforcing the participants' rights. Dr. Edward L. Raab stresses that knowledgeable agreement is a procedure that ought to be recorded, underscoring the significance of transparent communication.
Regulatory Compliance: Adhering to the ICF in clinical research guidelines is essential for meeting regulatory requirements, which is vital for the approval and ongoing conduct of clinical trials. Moreover, research shows that patients frequently recall only around 50% of what they were told during consent discussions, highlighting the necessity for effective communication.
In essence, ICFs transcend mere procedural formalities; they are critical for ethical involvement, safeguarding the rights of individuals, and fostering a culture of transparency and trust within clinical studies.
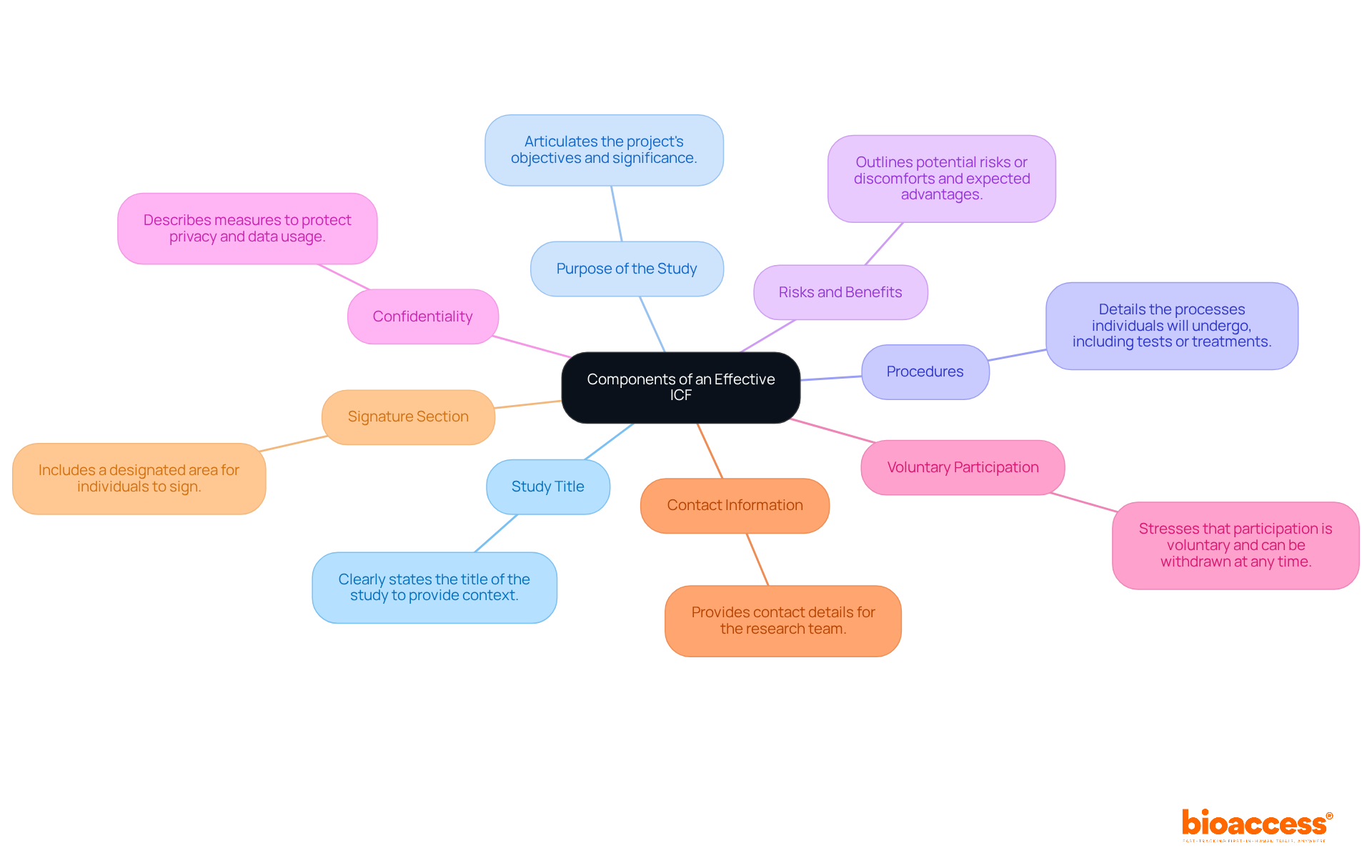
An effective ICF in clinical research must encompass several critical components to ensure individuals are thoroughly informed. These components include:
Including these elements not only guarantees adherence to ethical standards but also promotes trust and transparency between researchers and subjects, which is essential for the ICF in clinical research. Statistics indicate that many patients find ICFs overly technical, highlighting the need for clear and comprehensible language to enhance understanding. Best practices indicate that approval documents should be customized to the target population's needs, ensuring that all information is presented in an accessible manner. By prioritizing understanding among subjects, researchers can uphold ethical principles and encourage knowledgeable decision-making in clinical trials.
Acquiring knowledgeable approval presents numerous obstacles that researchers must confront to ensure ethical adherence and comprehension among participants. These key challenges include:
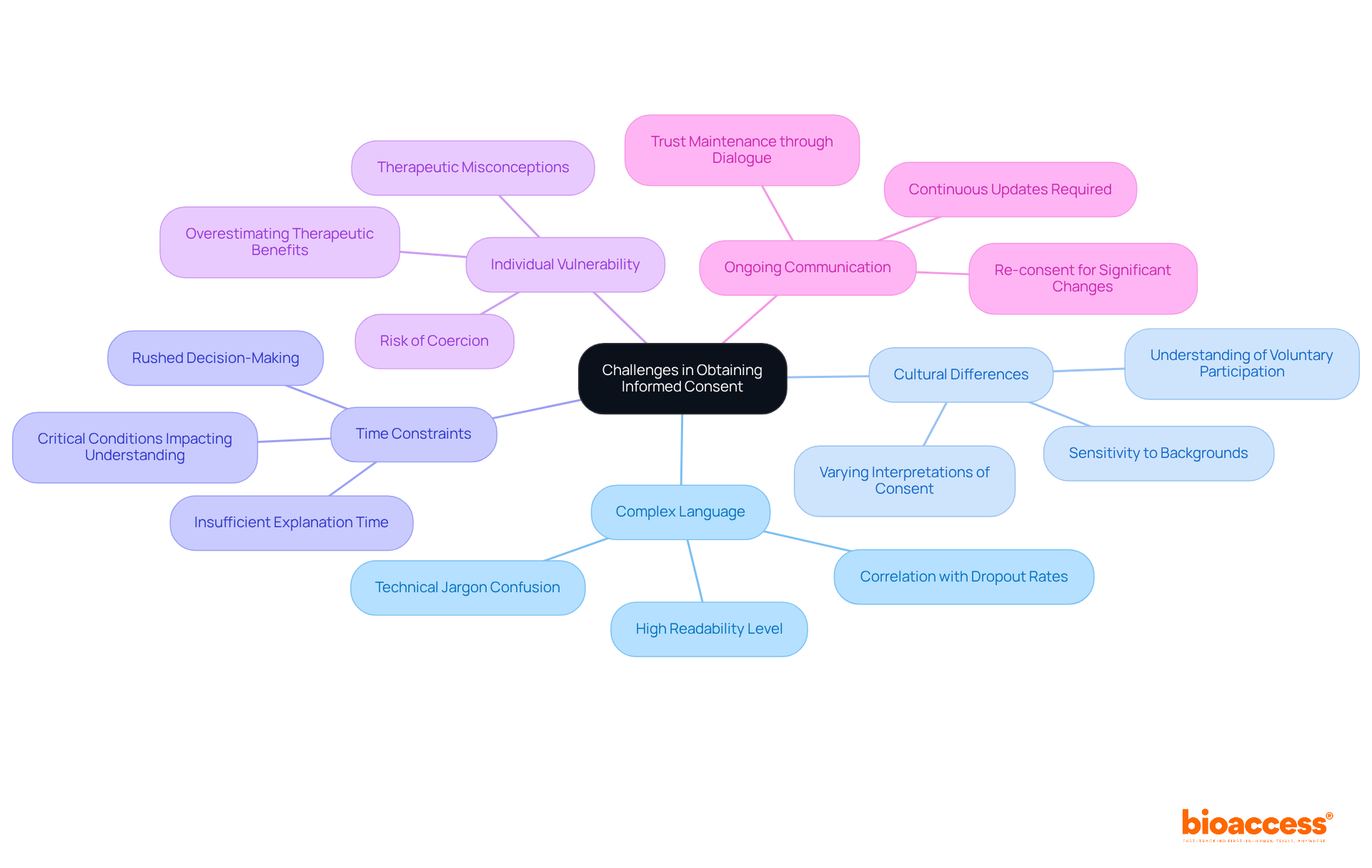
Complex Language: Many informed consent forms (ICFs) are laden with technical jargon, which can confuse participants. Research shows that the average readability of ICFs is at a high school level (Flesch-Kincaid Grade Level of 12.0), significantly exceeding the average reading level of adults. Simplifying language and employing everyday terminology in the ICF in clinical research can enhance understanding, as evidence suggests that clearer consent forms correlate with lower dropout rates.
Cultural Differences: Participants from diverse backgrounds may possess varying interpretations of consent and autonomy. For example, individuals from developing countries often exhibit different comprehension levels regarding the voluntary nature of participation and the freedom to withdraw. Researchers must be culturally sensitive and adapt their approach in line with ICF in clinical research to accommodate these differences, ensuring that all individuals feel respected and understood.
Time Constraints: In bustling clinical settings, there may be insufficient time to thoroughly explain the study. Clinical research nurses (CRNs) have noted that patients frequently face time-sensitive decisions due to critical conditions, jeopardizing the understanding process. Researchers should prioritize discussions about the ICF in clinical research and allocate adequate time for individuals to ask questions, fostering a more informed decision-making environment.
Individual Vulnerability: Some individuals may find themselves in vulnerable situations, rendering them more susceptible to coercion. It is crucial for researchers in clinical research to ensure that consent is obtained freely and without pressure, as outlined in the ICF in clinical research. This includes recognizing the potential for overestimating therapeutic benefits, particularly in critically ill patients, which can impact their voluntariness. Notably, 62.4% of individuals had no therapeutic misconceptions, underscoring the need for careful consideration in this domain.
Ongoing Communication: Informed consent is not a one-time event; it necessitates continuous communication. Researchers should provide updates and re-consent when significant changes occur in the study. The comprehension of informed consent elements has not improved over the last 30 years, highlighting the importance of ongoing dialogue to uphold trust and ensure that individuals remain aware of their rights and the study's progress.
By recognizing and addressing these challenges, researchers can enhance the informed consent process, ensuring that participants are genuinely informed and empowered to make autonomous decisions regarding their involvement in ICF in clinical research.
Informed Consent Forms (ICFs) transcend mere legal documents; they are the bedrock of ethical clinical research, ensuring that participants are thoroughly informed about the studies in which they choose to engage. The significance of ICFs is rooted in their capacity to uphold the autonomy and rights of individuals, fostering a culture of transparency and trust between researchers and participants.
Throughout this discussion, the key aspects of ICFs have been underscored, including:
Furthermore, the article has addressed the essential components of effective ICFs and the challenges researchers encounter in obtaining informed consent, such as:
Addressing these challenges is paramount for enhancing participant understanding and ensuring ethical compliance in clinical trials.
Ultimately, the significance of informed consent in clinical research is paramount. It is a vital process that empowers individuals to make informed decisions regarding their participation, reinforcing their rights and dignity. By prioritizing clear communication and continuous engagement, researchers can not only enhance the informed consent process but also contribute to the integrity and ethical standards of clinical research as a whole. Emphasizing the importance of ICFs will lead to improved participant experiences and more reliable clinical trial outcomes.
What are Informed Consent Forms (ICFs) in clinical research?
Informed Consent Forms (ICFs) are essential documents in clinical research that ensure individuals are fully informed about the studies they are considering. They outline the research's purpose, procedures, potential risks and benefits, and the rights of participants.
Why are ICFs important in clinical research?
ICFs are important because they serve as a legal affirmation of an individual's voluntary consent to participate in a study after they have received adequate information. They uphold the autonomy and rights of individuals involved in research.
What should an effective ICF include?
An effective ICF should be clear, concise, and tailored to the specific study context, enabling individuals to understand all relevant details before granting consent.
What challenges exist regarding participants' understanding of ICFs?
Research indicates that while about 75% of clinical trial volunteers understand various aspects of informed consent, comprehension of more complex elements, such as randomization and placebo concepts, has not improved over the past thirty years.
What recommendations are made to improve understanding of ICFs?
It is recommended to streamline consent documents and use innovative communication methods to enhance understanding among participants and ensure ethical standards are met.
How do reading levels of ICFs vary?
The SWOG agreement form was assessed at a 16th-grade reading level, while the LSU form was at a 7th-grade reading level, indicating significant readability challenges for participants.
What preference did participants show regarding ICF readability?
62% of individuals preferred the LSU form over the SWOG form, highlighting the need for simplifying consent documentation to improve participant comprehension.