


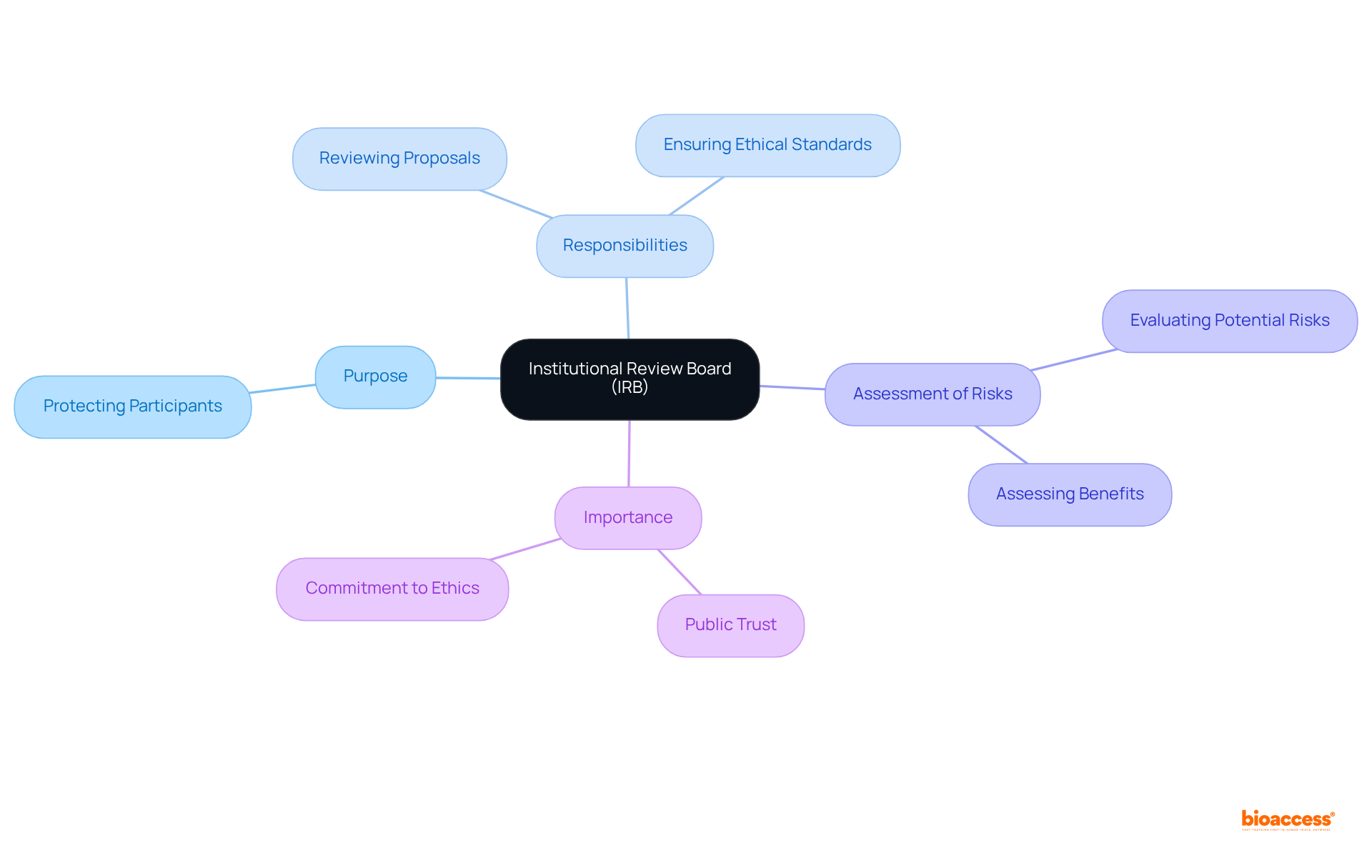
The article defines an Institutional Review Board (IRB) as a vital administrative body dedicated to safeguarding the rights and welfare of human research participants. It meticulously reviews research proposals to ensure ethical compliance. The significance of IRBs in upholding ethical standards, minimizing risks, and cultivating public trust in research is underscored. This is supported by their structured review processes and the increasing acknowledgment of their pivotal role within the scientific community.
Institutional Review Boards (IRBs) serve a pivotal function in maintaining ethical standards in research involving human subjects, acting as vigilant protectors of participant rights and welfare. By thoroughly reviewing study proposals, IRBs ensure that potential risks are minimized and that ethical compliance is upheld, thereby fostering public trust in scientific endeavors. Yet, as the research landscape continues to evolve, critical questions emerge: how can IRBs effectively balance rigorous oversight with the imperative for innovation? This exploration will:
An Institutional Review Board (IRB) serves as a pivotal administrative entity, established to protect the rights and welfare of human study participants. Its primary responsibility lies in reviewing research proposals to ensure adherence to ethical standards and regulatory requirements. The IRB meticulously assesses potential risks and benefits associated with studies, safeguarding participants' rights throughout the research process. This vital oversight not only preserves public trust in the research endeavor but also reinforces the commitment to upholding ethical principles.
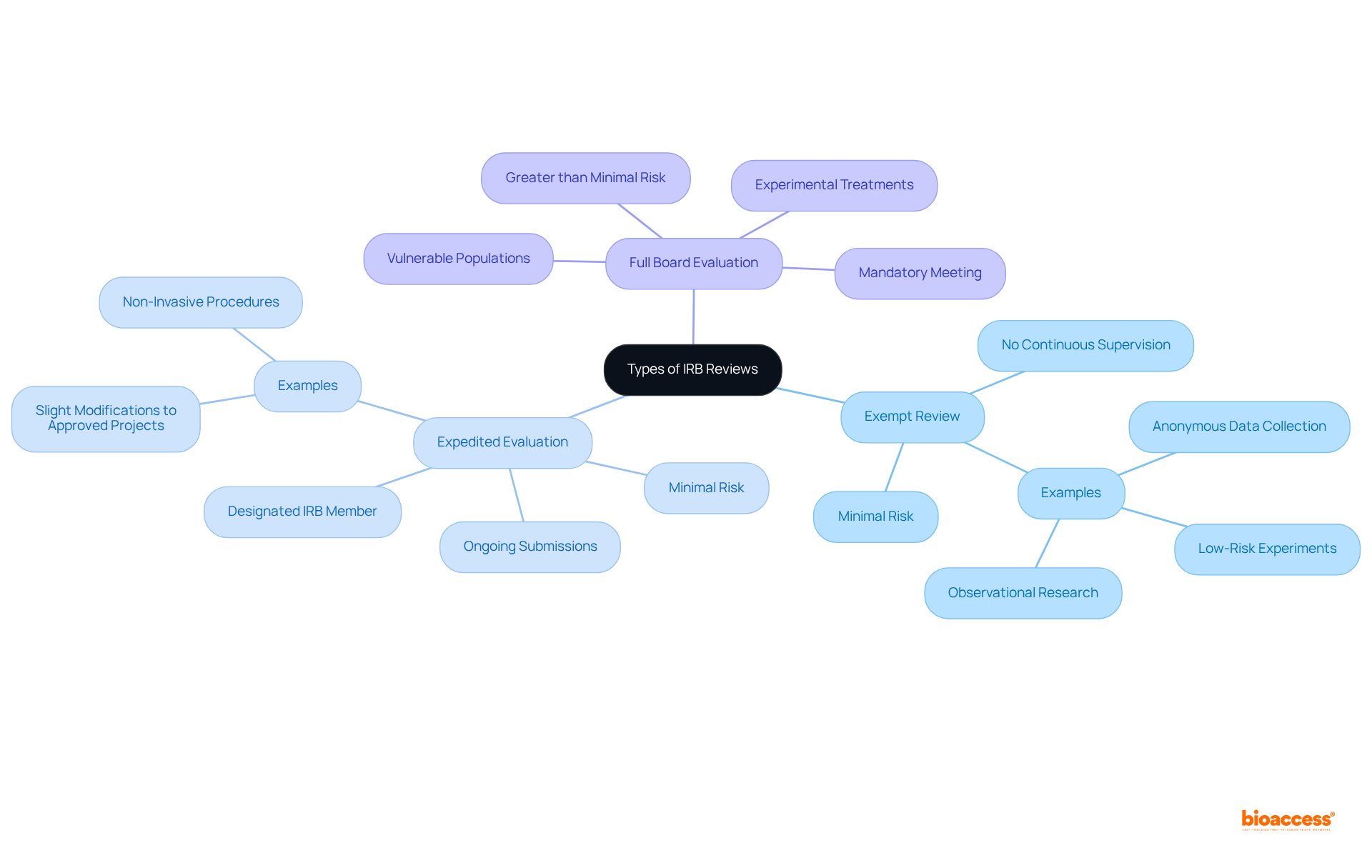
The internal review board definition encompasses three main types of reviews conducted by IRBs: exempt, expedited, and full board review, each serving distinct purposes in the approval process.
Exempt Review: This category applies to research posing minimal risk to participants and fitting specific federal definitions. Examples include anonymous data collection, low-risk experiments, and observational research. Research eligible for exempt evaluation does not require continuous supervision by the IRB, thereby simplifying the procedure for low-risk projects.
Expedited Evaluation: Designed for studies involving minimal risk, expedited evaluations can be conducted by a designated IRB member rather than the entire board. This category is frequently utilized for inquiries that involve slight modifications to previously sanctioned projects and necessitates continuous submissions to the IRB, facilitating swifter approvals and promoting faster data gathering and analysis.
Full Board Evaluation: This assessment is mandatory for research presenting more than minimal risk to participants, such as projects involving vulnerable populations or experimental treatments. It necessitates a convened meeting of the IRB, where all members discuss and evaluate the ethical considerations of the study prior to granting approval. Understanding the internal review board definition and the complete evaluation process is essential for researchers to prepare suitable submissions and anticipate assessment timelines.
Recent changes in IRB review processes have underscored the importance of clear documentation and adherence to institutional guidelines, ensuring that all studies involving human subjects are conducted ethically and efficiently. Researchers must prepare necessary documents for IRB submission, including a detailed study protocol and informed consent materials.
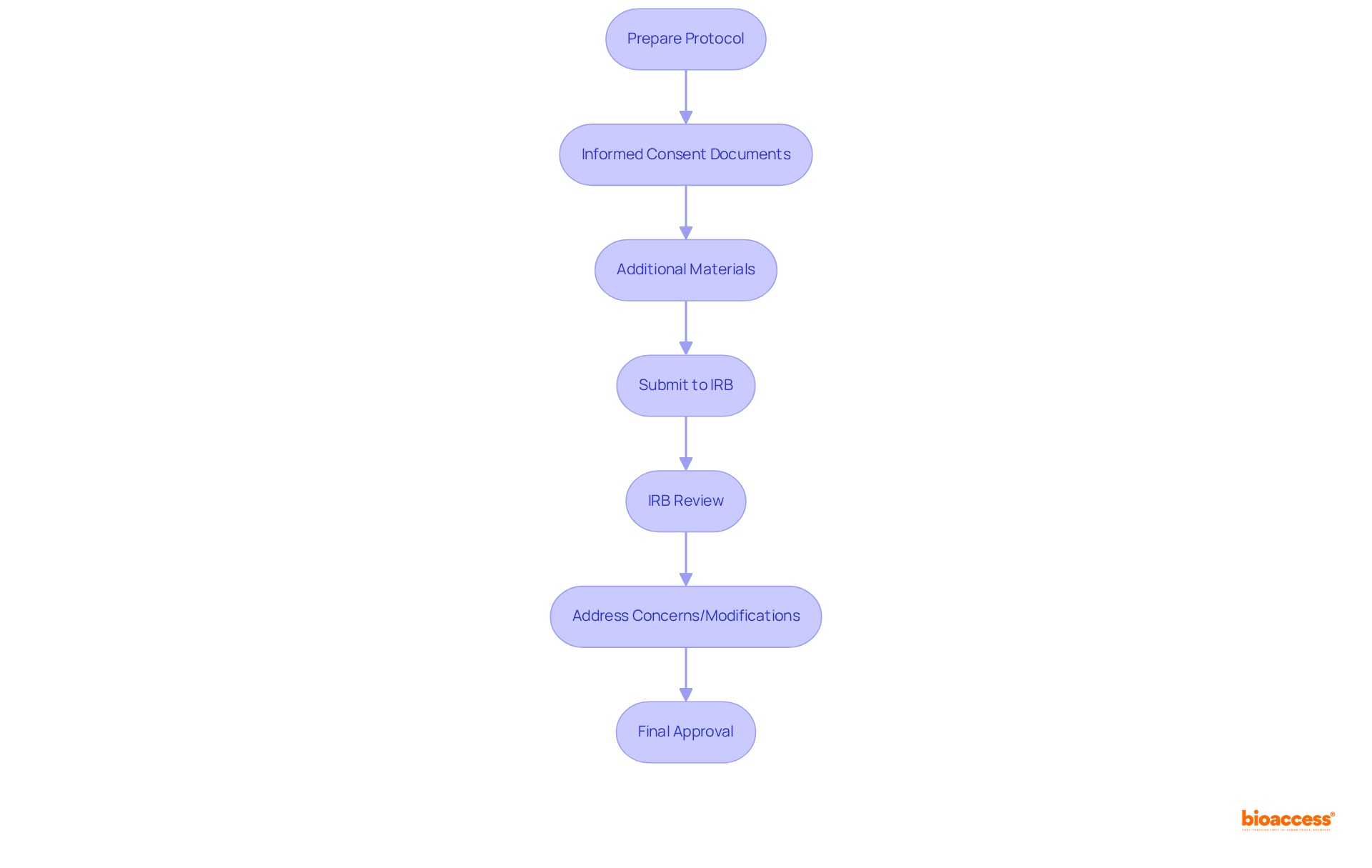
Presenting a proposal to an internal review board definition involves several essential steps and prerequisites that are crucial for ensuring ethical compliance in clinical studies. Researchers must begin by preparing a comprehensive protocol that clearly outlines the project's objectives, methodology, and potential risks to participants. This protocol serves as the foundation for the IRB's evaluation, establishing a clear framework for the review process.
In addition to the research protocol, informed consent documents are mandatory. These documents must effectively communicate the project's purpose, procedures, and any associated risks, empowering participants to make informed decisions about their involvement. Other necessary materials may include:
Once the submission is complete, the IRB will conduct a thorough examination of the provided materials. Researchers should be prepared to address any concerns or modifications requested by the board, as this iterative process is vital for achieving final approval. On average, ethical approvals can be delivered within 4 to 6 weeks—a timeframe that bioaccess® specifically achieves, significantly faster than traditional markets, thereby enhancing the overall timeline of clinical trials.
Successful IRB submissions underscore the importance of clear communication and meticulous documentation. Organizations with substantial expertise in clinical studies, such as bioaccess®, demonstrate that a well-prepared submission can lead to expedited evaluations and approvals. However, challenges may arise, including the need for additional information or clarifications, which can delay the process if not anticipated. As noted by clinical researchers, "The clarity of the submission can make a significant difference in the review process."
Overall, understanding the internal review board definition along with the submission requirements and processes is essential for researchers looking to navigate the complexities of clinical trials effectively.
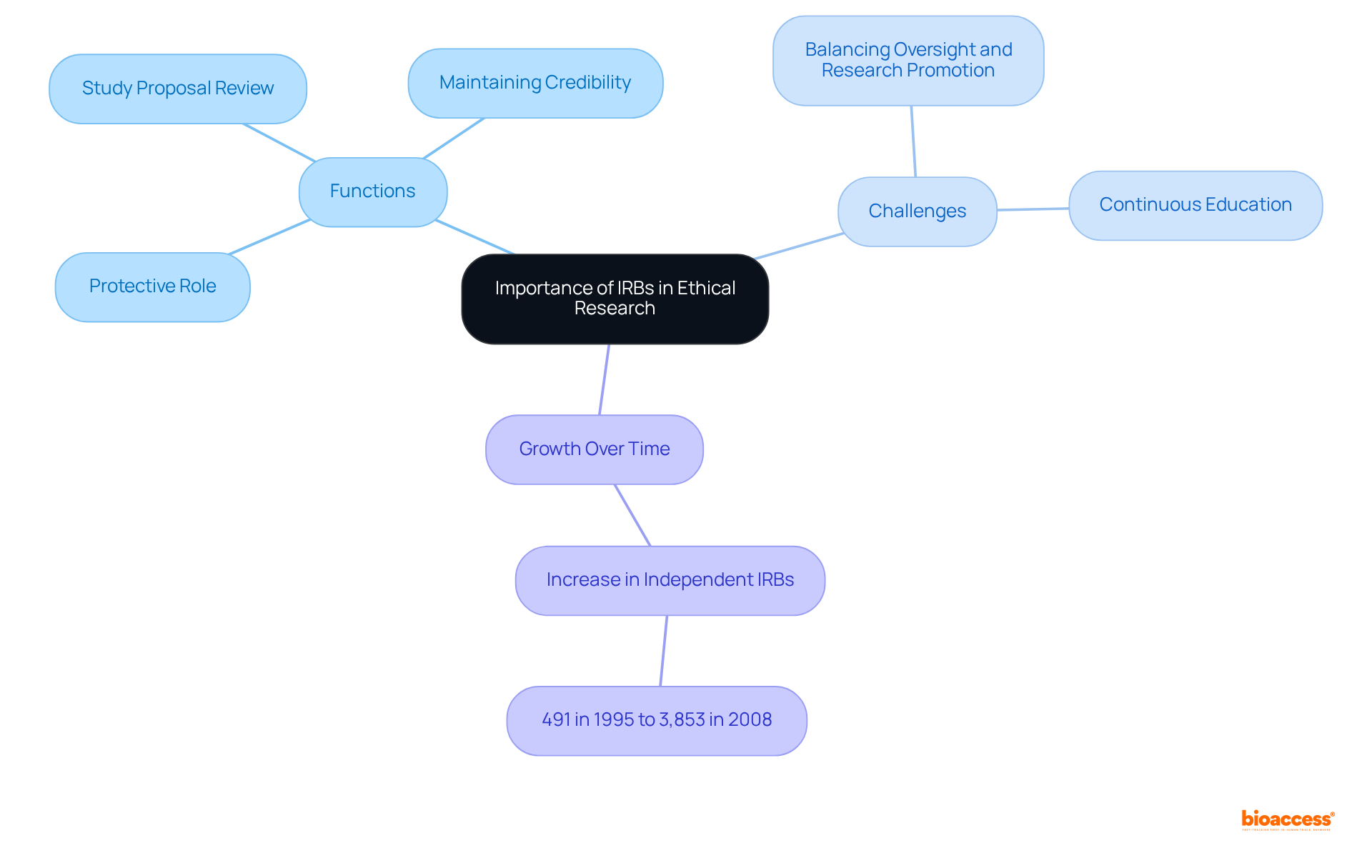
Institutional Review Boards (IRBs) serve a critical function in the realm of clinical research, ensuring that studies involving human subjects adhere to the highest ethical standards. Acting as a protective barrier against potential abuses, they guarantee that participants are treated with the utmost respect and dignity. Through meticulous review of study proposals, IRBs work diligently to minimize risks and enhance the ethical quality of research projects. This oversight not only safeguards participants but also bolsters the credibility and reliability of study results, which is essential for fostering public trust in the scientific community.
In an environment where ethical considerations are increasingly scrutinized, the importance of IRBs is paramount, standing as guardians of integrity and quality. Furthermore, IRBs conduct periodic reviews to assess study progress and ensure ongoing compliance with ethical standards. The increase in independent IRBs from 491 in 1995 to 3,853 in 2008 underscores the growing recognition of their significance in the scientific community.
However, IRBs face the challenge of balancing rigorous oversight with the promotion of research, a complexity that underscores their essential role. Continuous education and training are emphasized by IRBs to uphold high ethical standards, ensuring that all members are well-versed in current regulations and best practices. Notably, IRBs have played a pivotal role in preventing research misconduct, thereby reinforcing their essential function in maintaining research integrity.
Institutional Review Boards (IRBs) are pivotal in safeguarding the rights and welfare of human participants involved in research. By meticulously reviewing study proposals and ensuring compliance with ethical standards, IRBs serve as a crucial checkpoint in the research process, ultimately fostering public trust in scientific endeavors. Their unwavering commitment to ethical oversight not only protects participants but also enhances the credibility of research outcomes.
This article delves into the various types of IRB reviews—exempt, expedited, and full board evaluations—each tailored to address different levels of risk associated with research projects. It underscores the importance of clear documentation and adherence to submission requirements, which are vital for facilitating timely reviews. Furthermore, the discussion emphasizes how IRBs contribute to the integrity of research by conducting ongoing assessments and promoting ethical practices.
As the landscape of research continues to evolve, the significance of IRBs cannot be overstated. They stand as guardians of ethical standards, ensuring that the rights of participants are respected and that research is conducted with the utmost integrity. Researchers are encouraged to engage proactively with IRBs, gaining a comprehensive understanding of their processes and requirements to uphold ethical compliance effectively. By prioritizing ethical considerations, the scientific community can cultivate an environment of trust and reliability, ultimately advancing knowledge for the benefit of society.
What is an Institutional Review Board (IRB)?
An Institutional Review Board (IRB) is an administrative entity that protects the rights and welfare of human study participants.
What is the primary responsibility of an IRB?
The primary responsibility of an IRB is to review research proposals to ensure they adhere to ethical standards and regulatory requirements.
How does an IRB assess research studies?
An IRB meticulously assesses potential risks and benefits associated with studies to safeguard participants' rights throughout the research process.
Why is the oversight of an IRB important?
The oversight of an IRB is important as it preserves public trust in research endeavors and reinforces the commitment to upholding ethical principles.