


In the realm of clinical research, the standards defining investigator qualifications are not just bureaucratic hurdles; they are essential safeguards that uphold the integrity of studies and protect the welfare of participants. In Bosnia, where the landscape of medical inquiry is evolving yet grappling with significant challenges, grasping these criteria is crucial for researchers. This understanding is vital for navigating the complexities of compliance and ethics.
What implications do these qualifications hold for the future of clinical trials in Bosnia? How can they enhance the credibility and effectiveness of medical research in a region striving for improvement? These questions are not merely academic; they are pivotal for fostering a robust clinical research environment that can lead to meaningful advancements in healthcare.
Investigator qualification criteria in Bosnia are essential standards that individuals must meet to conduct research studies effectively. In Bosnia, the investigator qualification criteria are shaped by local regulations and international guidelines, ensuring that researchers possess the necessary skills to protect participant welfare and uphold research integrity. A qualified researcher typically holds a medical degree, undergoes specialized training in Good Clinical Practice (GCP), and demonstrates a solid track record in managing studies. This is particularly crucial in a context where Bosnia and Herzegovina has documented a total of 380 studies across both the Clinicaltrials.gov and Cortellis Clinical Trials Intelligence databases, yet still has the lowest number of studies per capita in the Balkans.
The enrollment procedure for research studies can take as long as 1.5 years, highlighting the administrative challenges faced by researchers. The significance of these qualifications cannot be overstated; they are vital for maintaining high standards in medical research and ensuring adherence to ethical and regulatory requirements. As industry specialists emphasize, effective investigator training and certification are crucial to the success of research studies, ultimately leading to improved outcomes for patients and the healthcare system as a whole.
Moreover, establishing a distinct ethical committee at the state level could expedite study registration, further enhancing the research environment in Bosnia. This collaborative effort is essential for addressing the key challenges in clinical research and fostering a more robust Medtech landscape.
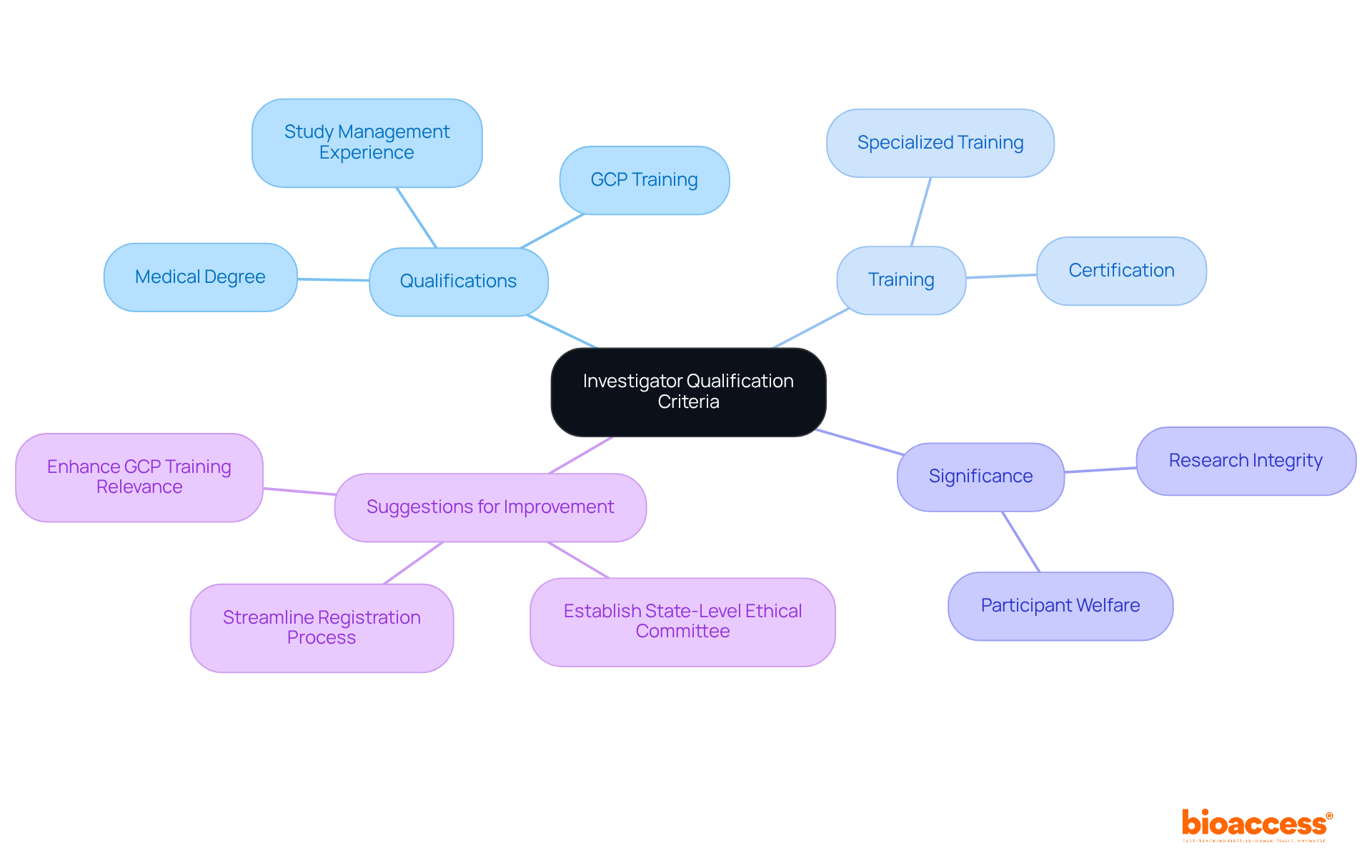
The investigator qualification criteria in Bosnia are crucial for maintaining high benchmarks in medical studies, particularly as the complexity of evaluations is on the rise. The investigator qualification criteria in Bosnia are vital for safeguarding human subjects and ensuring that studies are conducted with the highest ethical integrity. Investigators must not only have a profound understanding of the scientific principles behind their studies but also the capability to navigate the complex regulatory frameworks governing research involving human subjects, including adherence to ethical standards and oversight responsibilities.
The significance of these qualifications is underscored by their direct impact on the reliability of experimental data, which is essential for the approval of innovative therapies and medical devices. Research indicates that highly skilled researchers significantly enhance public confidence in clinical studies, a key factor for effective participant recruitment and retention. For instance, studies show that 93.6% of individuals with chronic conditions consider it crucial to know they can complete the entire study, emphasizing the need for proficient oversight throughout the research process.
Moreover, the ethical standards upheld by qualified investigators protect human subjects, ensuring their rights and well-being are prioritized. This commitment encompasses obtaining informed consent and implementing ongoing consent and re-consent processes, which are vital for participant engagement. Such ethical practices not only foster a positive environment for participants but also contribute to the overall success of research studies.
Bioaccess offers comprehensive trial management services, including:
These services are indispensable in supporting skilled researchers. As the landscape of medical inquiry continues to evolve, the emphasis on stringent qualifications for researchers remains essential for advancing medical science and enhancing patient care.
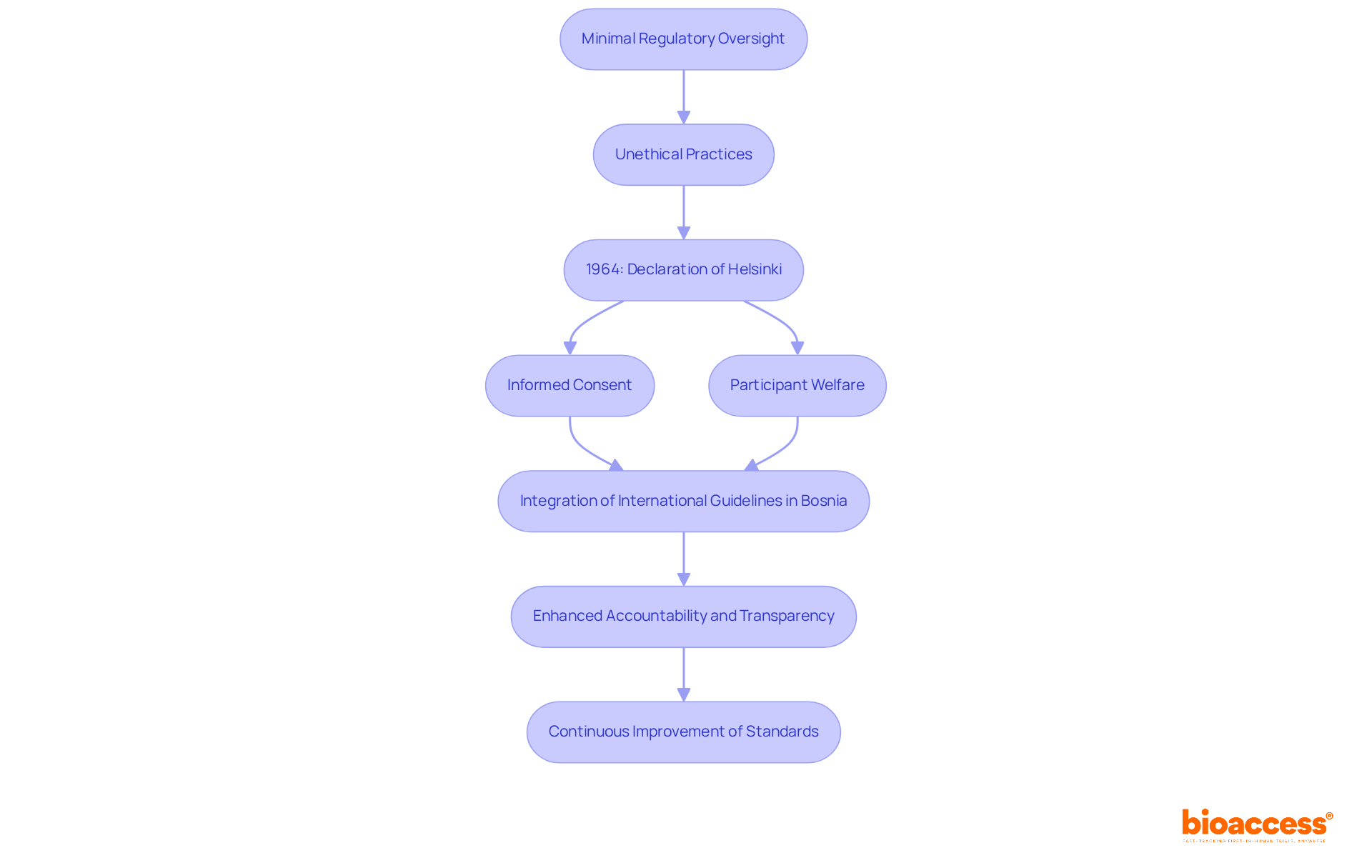
The development of researcher qualification standards in clinical studies stems from a time when regulatory oversight was minimal, often resulting in unethical practices that jeopardized patient safety. Landmark events, particularly the Declaration of Helsinki, have played a crucial role in reshaping these standards. First adopted in 1964, this declaration set forth ethical principles for medical studies involving human subjects, underscoring the importance of informed consent and participant welfare.
In Bosnia, the incorporation of international guidelines into local regulations has been vital for establishing the investigator qualification criteria in Bosnia. This integration signifies a global movement towards enhanced accountability and transparency in medical studies, ensuring that researchers possess the necessary expertise to conduct experiments that adhere to both ethical and scientific standards.
As the landscape of medical studies evolves, the continuous improvement of these standards becomes imperative. Addressing emerging challenges while safeguarding the integrity of the research process is essential for the future of clinical research.
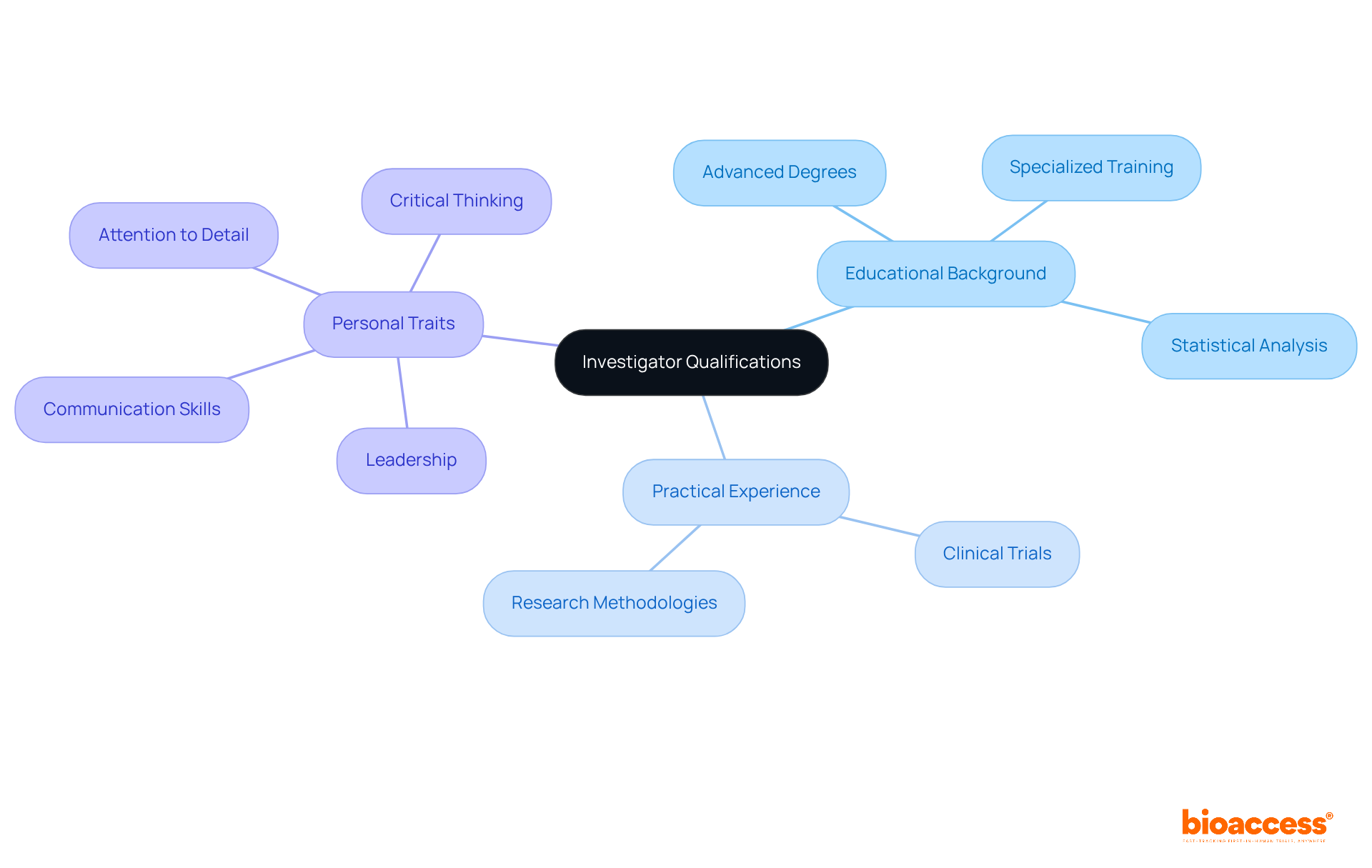
The credentials of research study leaders hinge on a blend of educational background, practical experience, and personal traits. Typically, these professionals hold advanced degrees in fields like medicine, pharmacology, or related disciplines, bolstered by specialized training in research methodologies. This educational foundation is crucial, equipping researchers with the skills needed to navigate the complexities of studies effectively.
In Bosnia, the investigator qualification criteria are increasingly acknowledged as a vital factor in the successful execution of clinical trials. Investigators are expected to demonstrate strong leadership abilities, effective communication skills, and unwavering dedication to ethical study practices. Personal attributes such as attention to detail, critical thinking, and the capacity to collaborate with diverse teams are essential for fostering a productive research environment.
Bioaccess® offers comprehensive clinical study management services that help researchers tackle challenges like the prolonged ethics committee approval process. Case studies illustrate the impact of educational qualifications on outcomes. For example, researchers with robust training in statistical analysis and trial design have been shown to enhance the reliability of results, ultimately improving patient care. Furthermore, integrating educational programs focused on medical studies in Bosnia is crucial for developing a skilled workforce that meets the investigator qualification criteria in Bosnia and can address the demands of contemporary medical inquiry. As the healthcare landscape evolves, prioritizing well-qualified investigators who meet the investigator qualification criteria in Bosnia will be pivotal in advancing clinical research and upholding ethical standards.
The investigator qualification criteria in Bosnia stand as a crucial foundation for conducting ethical and effective clinical research. These standards ensure that researchers possess the necessary skills and knowledge to protect participant welfare while upholding the integrity of the research process. By adhering to local regulations and international guidelines, Bosnia seeks to elevate the quality of its medical studies, ultimately enhancing patient care and fostering public trust in clinical research.
Key points throughout the article underscore the significance of having well-trained investigators equipped with advanced degrees, specialized training, and essential personal attributes. The challenges researchers face, such as lengthy enrollment procedures and the need for streamlined ethical oversight, highlight the critical importance of establishing robust qualification criteria. Furthermore, the historical context illustrates how past ethical failures have shaped current standards, reinforcing the ongoing need for improvement in the research landscape.
As Bosnia continues to develop its clinical research environment, the demand for well-qualified investigators becomes increasingly vital. Investing in education and training programs will not only enhance researchers' capabilities but also cultivate a culture of ethical responsibility and transparency. By prioritizing these qualifications, Bosnia can ensure that its clinical trials contribute meaningfully to the advancement of medical science and the well-being of patients.
What are investigator qualification criteria in Bosnia?
Investigator qualification criteria in Bosnia are essential standards that individuals must meet to conduct research studies effectively, shaped by local regulations and international guidelines to ensure researchers have the necessary skills to protect participant welfare and uphold research integrity.
What qualifications do researchers typically need in Bosnia?
A qualified researcher in Bosnia typically holds a medical degree, has undergone specialized training in Good Clinical Practice (GCP), and demonstrates a solid track record in managing studies.
How many studies have been documented in Bosnia and Herzegovina?
Bosnia and Herzegovina has documented a total of 380 studies across both the Clinicaltrials.gov and Cortellis Clinical Trials Intelligence databases.
What challenges do researchers face in Bosnia regarding study enrollment?
The enrollment procedure for research studies in Bosnia can take as long as 1.5 years, highlighting administrative challenges faced by researchers.
Why are investigator qualifications important in medical research?
Investigator qualifications are vital for maintaining high standards in medical research, ensuring adherence to ethical and regulatory requirements, and ultimately leading to improved outcomes for patients and the healthcare system.
What could improve the research environment in Bosnia?
Establishing a distinct ethical committee at the state level could expedite study registration and enhance the research environment in Bosnia, addressing key challenges in clinical research and fostering a more robust Medtech landscape.