


Isotype immunoglobulins, or classes of antibodies, are essential components of the immune system, consisting of five primary types—IgA, IgD, IgE, IgG, and IgM. Each type serves distinct functions in immune responses, underscoring their critical roles. This article highlights their importance by discussing how these isotypes adapt to various pathogens and their contributions to long-term immunity. Furthermore, it delves into their clinical implications in therapies, emphasizing the necessity of understanding their dynamics for effective immunological interventions.
Understanding the intricacies of isotype immunoglobulins reveals a compelling landscape of the immune system's defense mechanisms. These classes of antibodies—IgA, IgD, IgE, IgG, and IgM—each play unique and vital roles in shaping the body's response to pathogens and maintaining health.
However, the challenges surrounding the measurement and interpretation of these immunoglobulin levels in clinical practice raise crucial questions about their true impact on immunity and disease management.
Delving into these questions not only enhances our understanding but also underscores the importance of innovative approaches in clinical research.
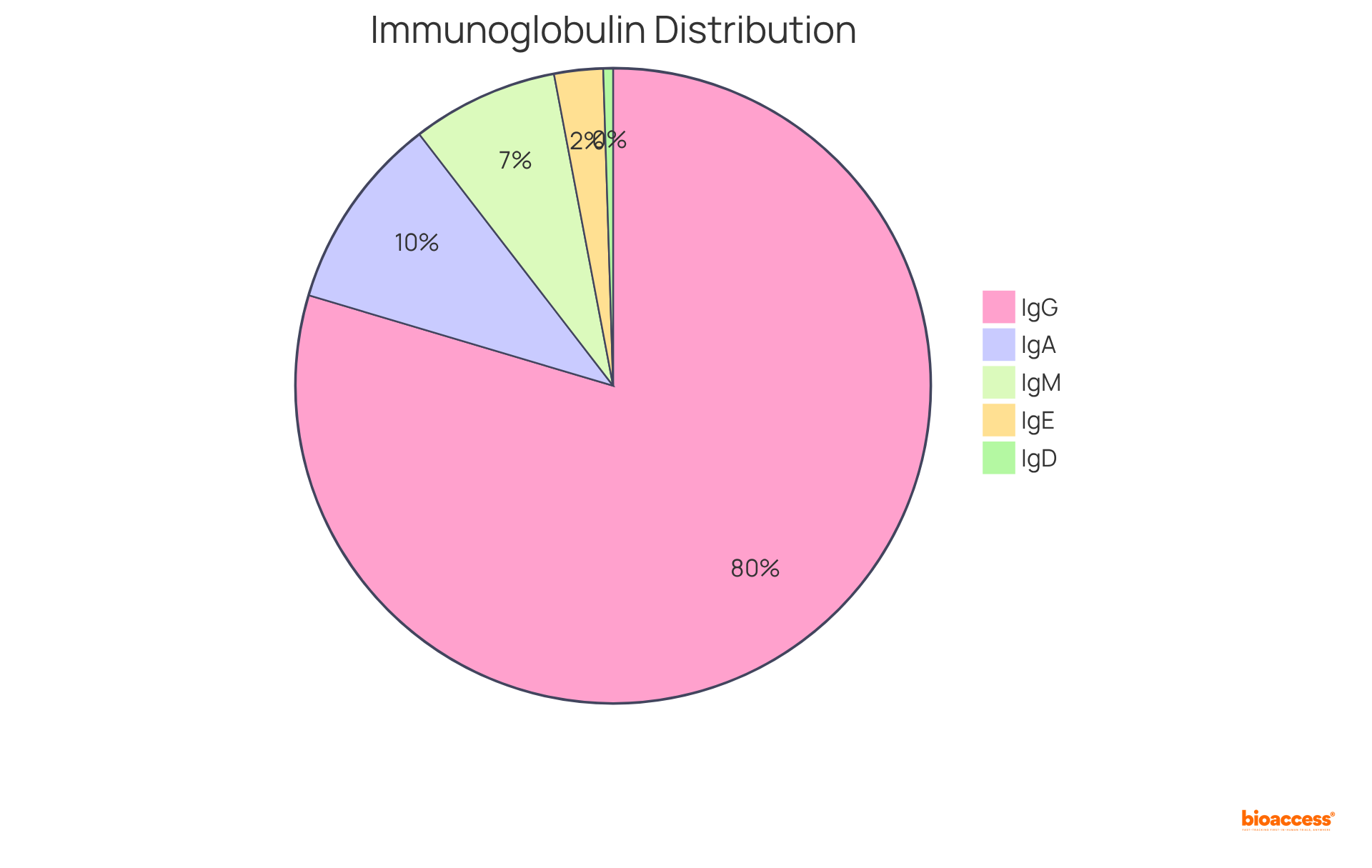
Isotype immunoglobulin, which are commonly referred to as classes of antibodies, represent distinct categories that are crucial to the immune system. In humans, five primary types exist: IgA, IgD, IgE, IgG, and IgM. Each isotype immunoglobulin is characterized by its unique heavy chain structure, which dictates its function and distribution throughout the body. Notably, IgD constitutes less than 1% of the total plasma immunoglobulin, whereas IgG is the predominant isotype immunoglobulin in serum, accounting for approximately 70-85% of the total immunoglobulin pool, playing a vital role in long-term immunity. Conversely, the isotype immunoglobulin IgM, which makes up about 5-10% of the immunoglobulin pool, is the first antibody produced in response to an infection. Understanding isotype immunoglobulin variants is essential for comprehending how the body’s defense system adapts to various pathogens and maintains equilibrium.
Recent studies have underscored the importance of isotype immunoglobulin variants in adapting to different pathogens. For instance, research reveals that individuals with deficiencies in isotype immunoglobulin subclasses, particularly IgG, may face heightened susceptibility to infections, highlighting the necessity of measuring these subclasses in clinical contexts. Furthermore, the correlation between elevated isotype immunoglobulin levels and improved resistance in severe COVID-19 cases illustrates the critical role of isotype immunoglobulin in long-term defensive responses. As emphasized by S. Sayyah, the interplay between isotype immunoglobulin IgM and IgG proteins in individuals affected by severe acute respiratory syndrome coronavirus further underscores the relevance of these variants in immune responses. Additionally, many children with a subclass deficiency in early childhood achieve normal subclass levels as they mature, demonstrating the adaptable nature of antibodies and their significance for health. However, it is crucial to recognize that measuring isotype immunoglobulin levels, particularly IgG subclass levels, is not universally advised for assessing antibody-mediated immunity in individuals with recurrent infections, as 2.5% of healthy individuals may be classified as deficient based on standard range values. Understanding the dynamics of isotype immunoglobulin categories is vital for developing effective immunotherapies and vaccines, as well as for evaluating immune defenses across diverse populations. This knowledge not only propels medical research forward but also enhances clinical practices in managing immunodeficiencies and infectious diseases.
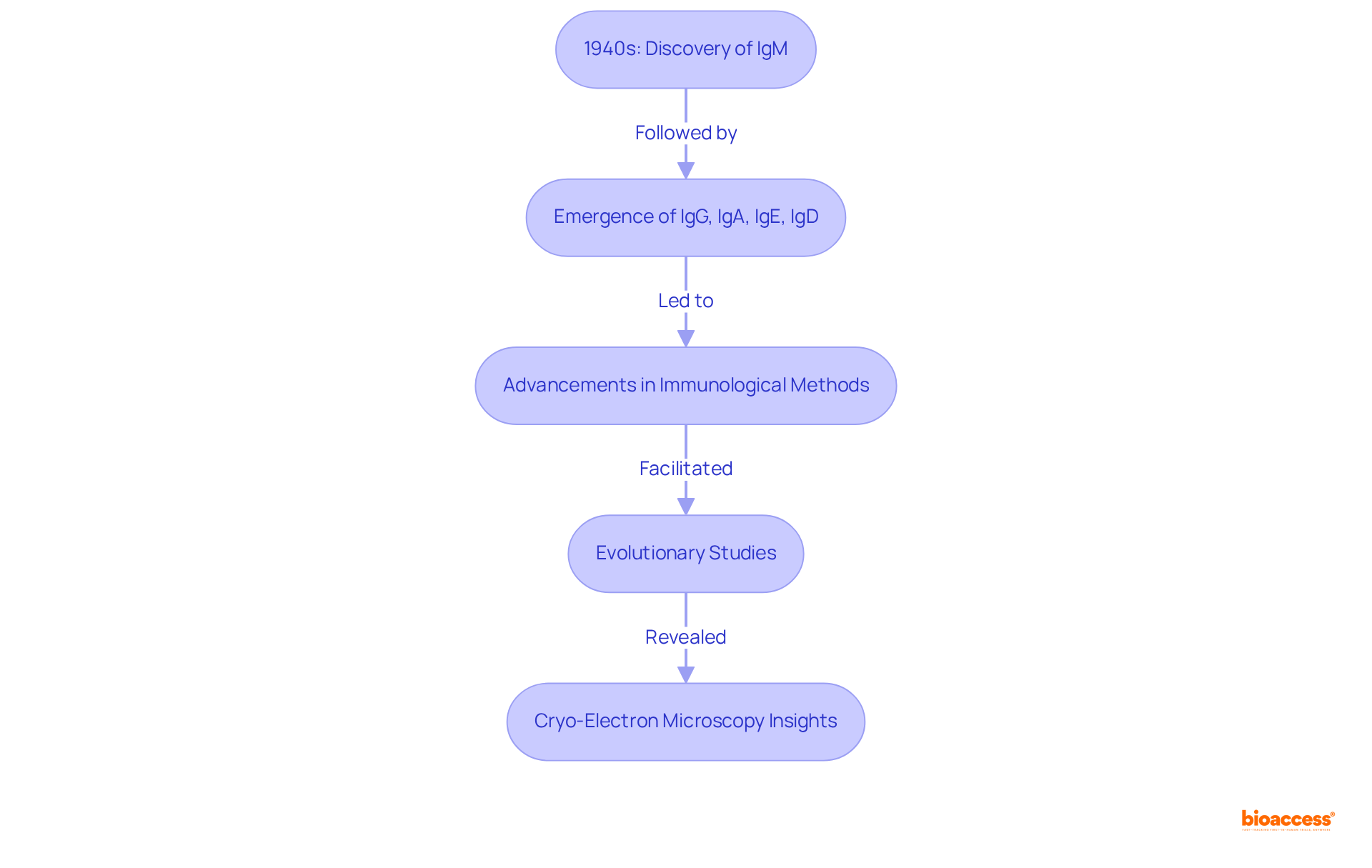
The categorization of isotype immunoglobulin types emerged in the mid-20th century, as researchers began to distinguish different classes of immune proteins. The pivotal discovery of IgM in the 1940s marked a significant milestone, underscoring its crucial role in the primary defense response and its status as the most ancient antibody isotype, originating around 500 million years ago. This was succeeded by the identification of other antibody types: IgG, IgA, IgE, and IgD, each characterized by unique structural features and immune functions. Notably, IgM and IgE possess a total of four heavy chain constant domains, one more than other antibody types.
The advancement of immunological methods, particularly immunoelectrophoresis and enzyme-linked immunosorbent assays (ELISA), significantly enhanced our understanding of these variants. These methodologies facilitated their classification and enabled comprehensive studies across diverse clinical applications. Furthermore, evolutionary studies contribute to predicting protein structures and understanding functional constraints, while cryo-electron microscopy structures have revealed that a joining chain is fundamental for the assembly of polymeric Ig forms.
This historical context illustrates the critical importance of isotype immunoglobulin variants in both fundamental and applied immunology, shaping our approach to disease diagnosis and treatment.
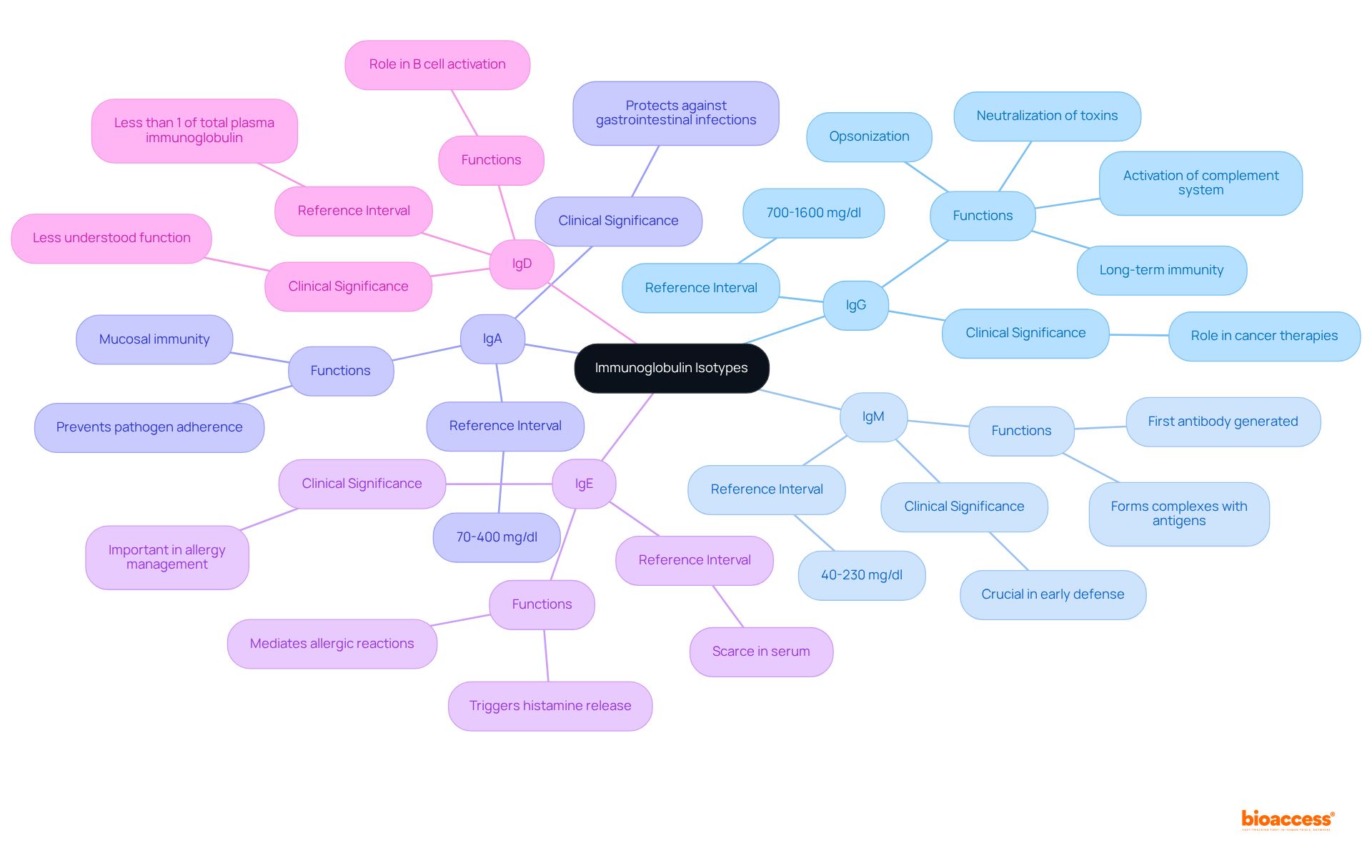
Immunoglobulin isotypes can be categorized based on their unique functions:
IgG: The most prevalent antibody in serum, IgG is vital for long-term immunity and can cross the placenta, providing passive immunity to the fetus. It is involved in opsonization, neutralization of toxins, and activation of the complement system. Clinical effectiveness statistics highlight its significant role in cancer therapies, where it has been shown to enhance therapeutic outcomes.
IgM: This isotype is the first antibody generated during a defense response and is effective in forming complexes with antigens, leading to their removal. Its pentameric structure enables strong attachment to pathogens, positioning it as a crucial factor in the early phases of defense.
IgA: Predominantly found in mucosal areas and secretions (such as saliva and breast milk), IgA plays a crucial role in mucosal immunity by preventing pathogen adherence to epithelial cells. Clinical examples underscore its importance in gastrointestinal and respiratory health, particularly in protecting against infections.
IgE: Associated with allergic reactions and responses to parasitic infections, IgE binds to allergens and triggers histamine release from mast cells. Its role in mediating hypersensitivity reactions underscores its clinical significance in allergy management.
IgD: Although its exact function remains less understood, IgD is primarily found on the surface of B cells and is believed to play a role in B cell activation and differentiation. Its presence suggests a role in the initial phases of the body's defense response, although additional research is required to completely clarify its functions.
Each isotype immunoglobulin possesses unique properties that enable the immune system to mount tailored responses to a diverse array of pathogens. Reference intervals for serum immunoglobulins in healthy adults are established as follows: IgA 70-400 mg/dl, IgG 700-1600 mg/dl, and IgM 40-230 mg/dl. Understanding these values is crucial for interpreting immunoglobulin levels in clinical practice, especially considering factors such as age, sex, and lifestyle that can influence these levels.
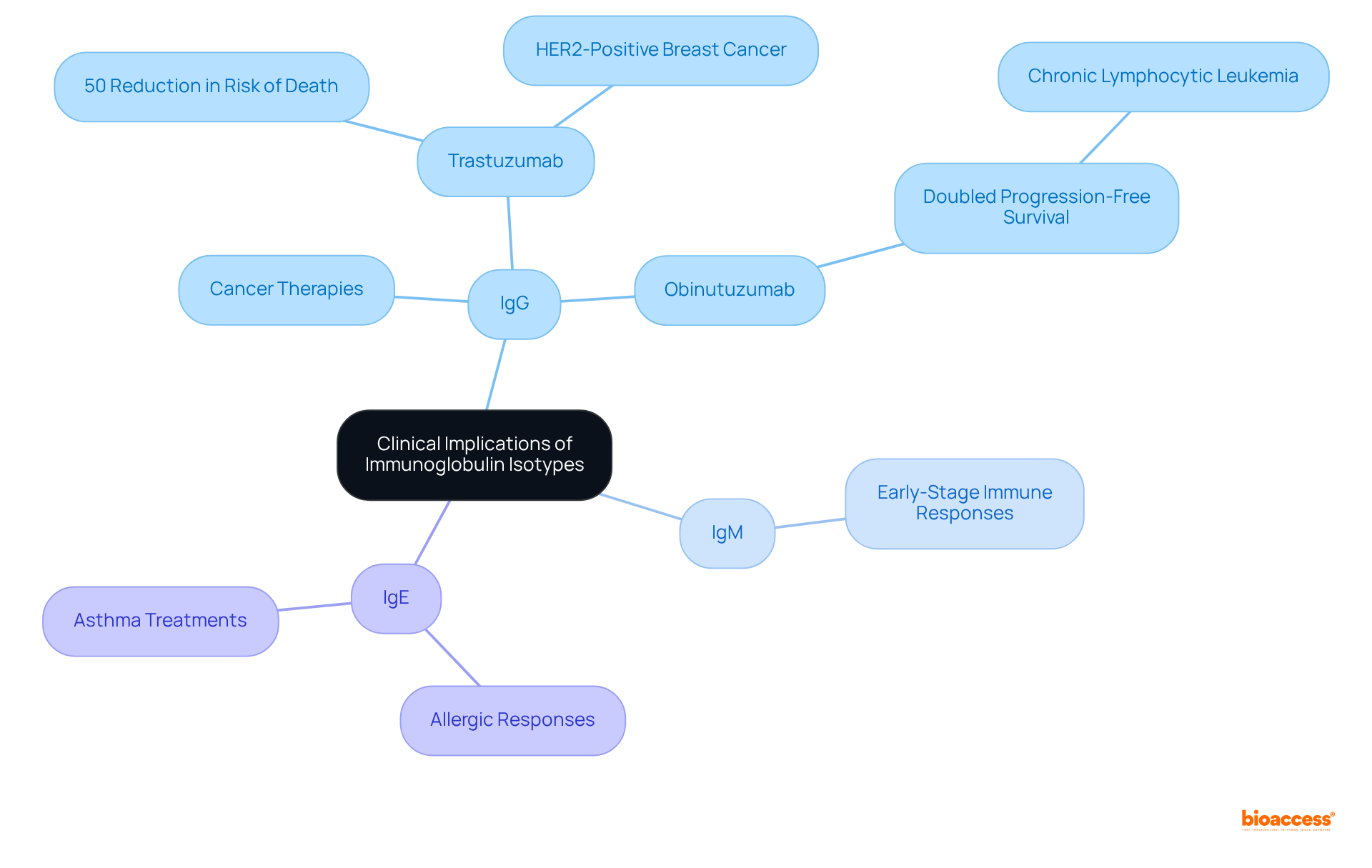
The clinical consequences of isotype immunoglobulin types are paramount, particularly within the realm of monoclonal treatments. The selection of isotype immunoglobulin can significantly influence the effectiveness of therapeutic proteins. For instance, the isotype immunoglobulin IgG, especially IgG1, is frequently employed in cancer therapies due to its robust capacity to engage immune effector functions, which is essential for effective tumor targeting.
Therapeutic proteins such as trastuzumab, an IgG1, have been shown to reduce the risk of death by 50% for patients with HER2-positive breast cancer. Currently, ninety-one monoclonal proteins are in clinical use for cancer therapy, highlighting the critical importance of isotype immunoglobulin selection in treatment strategies.
Furthermore, the glycoengineered anti-CD20 obinutuzumab (GA101) has nearly doubled progression-free survival rates in chronic lymphocytic leukemia patients compared to rituximab, illustrating the impact of antibody modifications on treatment efficacy. Conversely, IgM variants are under investigation for their potential to trigger early-stage immune responses, which could be beneficial in specific therapeutic contexts.
Additionally, the role of IgE in allergic responses has spurred the development of targeted treatments for conditions such as asthma and allergic rhinitis, showcasing the versatility of immunoglobulin classes in therapeutic applications. Ongoing research into the functional diversity of the isotype immunoglobulin continues to shape the design of innovative therapeutics, enhancing their specificity and effectiveness across a spectrum of diseases.
Isotype immunoglobulins are crucial components of the immune system, functioning as distinct classes of antibodies that fortify the body's defenses against pathogens. Each isotype—IgA, IgD, IgE, IgG, and IgM—possesses unique structural characteristics and functions, allowing the immune system to customize its responses to a diverse array of threats. Understanding isotype immunoglobulins is vital for comprehending how the immune system adapts and evolves in response to infections and diseases.
The article provided significant insights into the historical development, functions, and clinical implications of immunoglobulin isotypes. The evolution of these antibodies has been underscored by major discoveries, including the identification of IgM as the first antibody produced during an immune response and the essential role of IgG in long-term immunity and therapeutic applications. Furthermore, it emphasized the necessity of measuring isotype immunoglobulin levels in clinical contexts, particularly for understanding immune deficiencies and customizing effective treatments.
Recognizing the importance of isotype immunoglobulins transcends academic understanding; it bears significant implications for medical research and therapeutic strategies. As advancements in immunology continue to progress, the potential for developing targeted treatments based on isotype functions will enhance disease management and improve patient outcomes. Embracing this knowledge not only empowers healthcare professionals but also highlights the intricate and adaptive nature of the immune system in safeguarding health.
What are isotype immunoglobulins?
Isotype immunoglobulins, or classes of antibodies, are distinct categories that play a crucial role in the immune system. In humans, there are five primary types: IgA, IgD, IgE, IgG, and IgM, each characterized by its unique heavy chain structure.
What is the function of IgG?
IgG is the predominant isotype immunoglobulin in serum, accounting for approximately 70-85% of the total immunoglobulin pool. It plays a vital role in long-term immunity.
What role does IgM play in the immune response?
IgM constitutes about 5-10% of the immunoglobulin pool and is the first antibody produced in response to an infection.
Why is understanding isotype immunoglobulin variants important?
Understanding isotype immunoglobulin variants is essential for comprehending how the body’s defense system adapts to various pathogens and maintains equilibrium.
What can deficiencies in isotype immunoglobulin subclasses indicate?
Deficiencies in isotype immunoglobulin subclasses, particularly IgG, may lead to heightened susceptibility to infections, highlighting the importance of measuring these subclasses in clinical contexts.
How do isotype immunoglobulins relate to COVID-19?
Elevated isotype immunoglobulin levels have been correlated with improved resistance in severe COVID-19 cases, illustrating their critical role in long-term defensive responses.
What is the significance of IgM and IgG interplay during infections?
The interplay between isotype immunoglobulin IgM and IgG proteins is relevant in immune responses, particularly in individuals affected by severe acute respiratory syndrome coronavirus.
Can children with subclass deficiencies achieve normal levels as they mature?
Yes, many children with a subclass deficiency in early childhood can achieve normal subclass levels as they mature, demonstrating the adaptable nature of antibodies.
Is measuring isotype immunoglobulin levels always advised for recurrent infections?
Measuring isotype immunoglobulin levels, especially IgG subclass levels, is not universally advised for assessing antibody-mediated immunity in individuals with recurrent infections, as 2.5% of healthy individuals may be classified as deficient based on standard range values.
How does knowledge of isotype immunoglobulin dynamics benefit medical research and clinical practice?
Understanding the dynamics of isotype immunoglobulin categories is vital for developing effective immunotherapies and vaccines, as well as for evaluating immune defenses across diverse populations, enhancing clinical practices in managing immunodeficiencies and infectious diseases.