


The abbreviation 'mabs' signifies monoclonal antibodies, which are engineered proteins designed to replicate the immune system's capacity to identify and combat specific pathogens and diseases. This article underscores their critical importance in the field of medicine, particularly within cancer treatment and immunotherapy. Monoclonal antibodies have demonstrated a remarkable ability to enhance patient outcomes while minimizing side effects when compared to conventional therapies. Their role in clinical research is pivotal, as they represent a significant advancement in therapeutic strategies aimed at improving patient care.
Monoclonal antibodies, commonly referred to as "mabs," signify a monumental advancement in medical science, fundamentally transforming the diagnosis and treatment of diseases. These engineered proteins emulate the immune system's inherent capability to identify harmful pathogens, providing targeted solutions for a spectrum of conditions, including cancers and autoimmune disorders.
As the realm of monoclonal therapies broadens, what challenges and considerations emerge in their implementation and development?
Delving into the significance of mabs not only highlights their transformative impact on patient care but also uncovers the complexities that accompany their integration into contemporary medicine.
The mabs medical abbreviation refers to monoclonal proteins—laboratory-engineered molecules that replicate the immune system's ability to combat harmful pathogens. These proteins are specifically designed to bind to particular antigens on cell surfaces, making them essential in diagnosing and managing a range of conditions, including:
Derived from a single clone of a unique white blood cell, monoclonal proteins ensure uniformity, targeting the same epitope, or specific segment of the antigen.
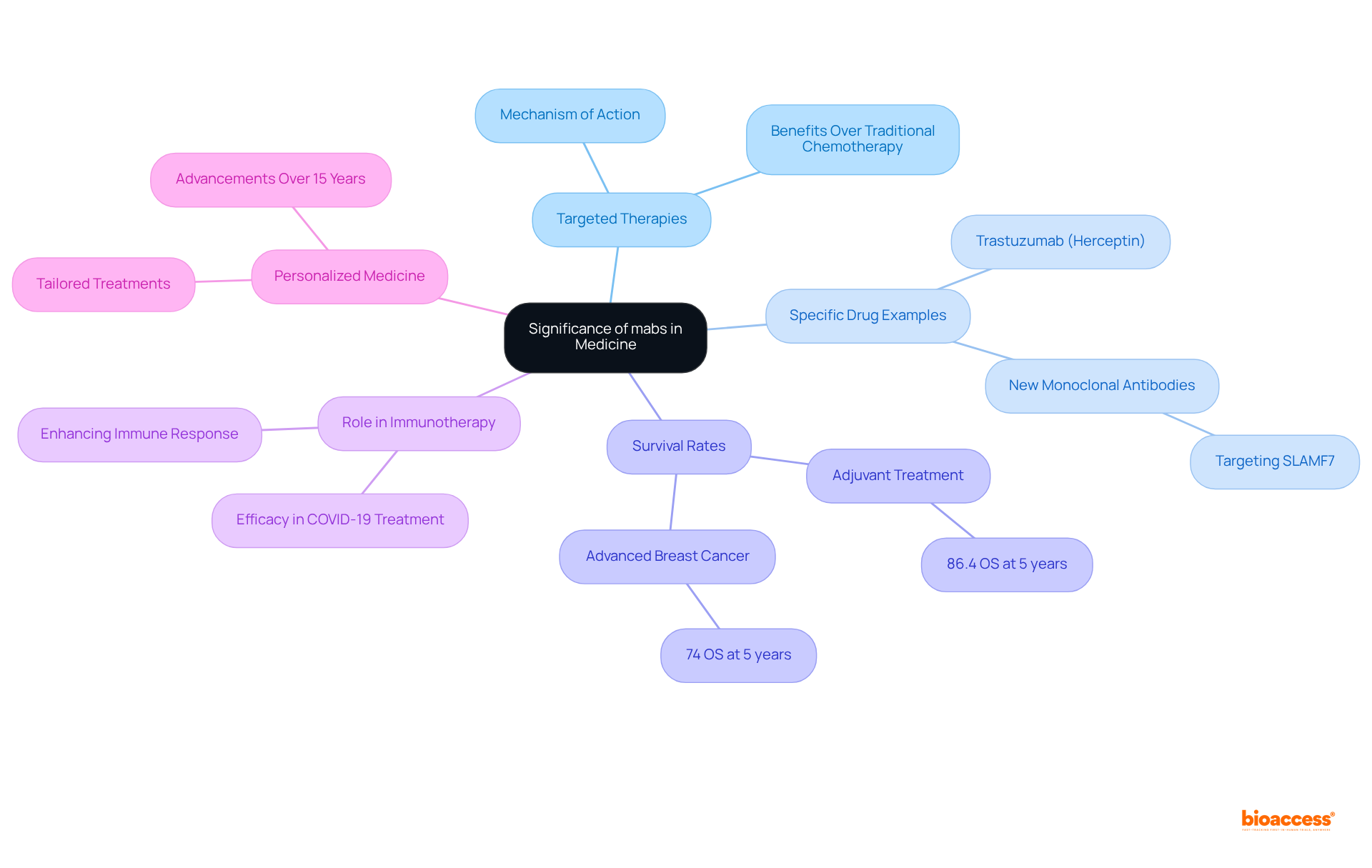
The mabs medical abbreviation represents monoclonal antibodies, which have fundamentally transformed the landscape of medicine, particularly in oncology and immunology. These targeted therapies, which are part of the mabs medical abbreviation, are meticulously designed to specifically attack cancer cells while preserving healthy cells, significantly reducing the side effects commonly associated with traditional chemotherapy.
For instance, trastuzumab (Herceptin) has been crucial in addressing HER2-positive breast cancer, with five-year overall survival rates reaching an impressive 86.4% for patients undergoing adjuvant care. This statistic underscores the drug's effectiveness in improving patient outcomes for this specific group. Furthermore, the median overall survival (OS) at five years for all patients with advanced breast cancer stands at 74%, emphasizing the broader influence of monoclonal antibodies in cancer care.
Monoclonal antibodies, commonly known by the mabs medical abbreviation, also play a vital role in immunotherapy, enhancing the immune system's capacity to combat pathogens, which has proven essential in treating infections, including COVID-19. Their application in managing COVID-19 has demonstrated significant efficacy in reducing disease severity.
The ability of these therapies to engineer specificity allows for personalized medicine approaches, tailoring treatments to meet the unique needs of individual patients, thereby maximizing therapeutic efficacy. Over the past 15 years, advancements in personalized medicine have further refined the application of monoclonal antibodies, ensuring that treatments are not only more effective but also finely attuned to individual patient characteristics.
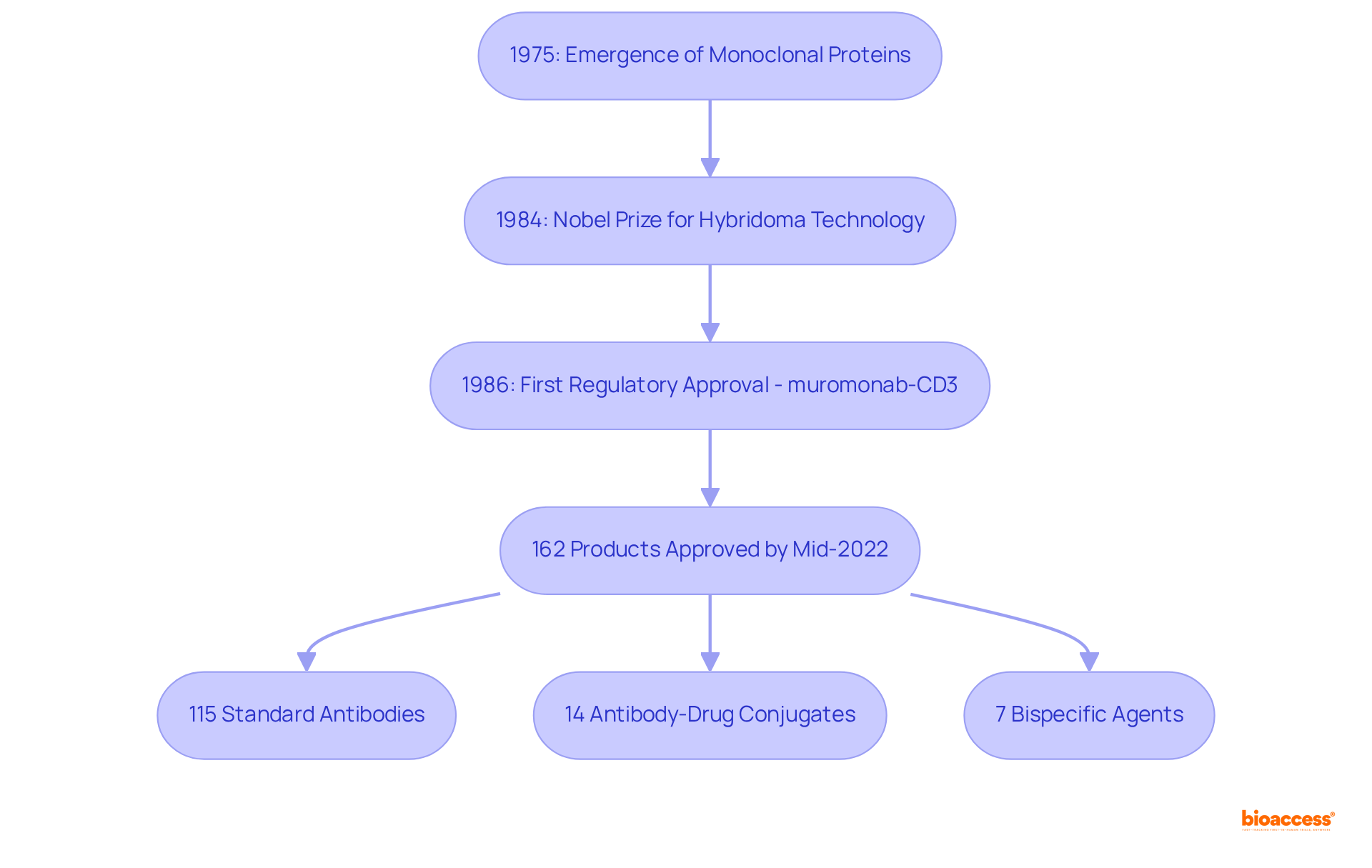
Monoclonal proteins (mAbs) emerged as a revolutionary concept in 1975, thanks to the pioneering work of Georges Köhler and César Milstein, who developed hybridoma technology. This innovative method allows for the creation of identical proteins from a single clone of B cells, a breakthrough that earned them the Nobel Prize in Physiology or Medicine in 1984.
The first monoclonal agent to gain regulatory approval for clinical use was muromonab-CD3 (Orthoclone OKT3) in 1986, specifically designed to prevent organ transplant rejection. Since that initial approval, the landscape of monoclonal therapies has expanded significantly, with over 162 monoclonal products receiving regulatory approval for various therapeutic applications by mid-2022. Notably, the United States leads with 122 approvals, followed by Europe with 114, reflecting the global impact of these therapies.
The authorized therapies consist of:
This highlights the variety of options available for care. The COVID-19 pandemic further accelerated progress in this area, with the swift authorization of neutralizing agents like Bamlanivimab, emphasizing the vital role of mAbs medical abbreviation in modern medicine. This exceptional growth underscores the progression of care alternatives across various disease fields, mirroring the continuous improvements in immune protein research and development.
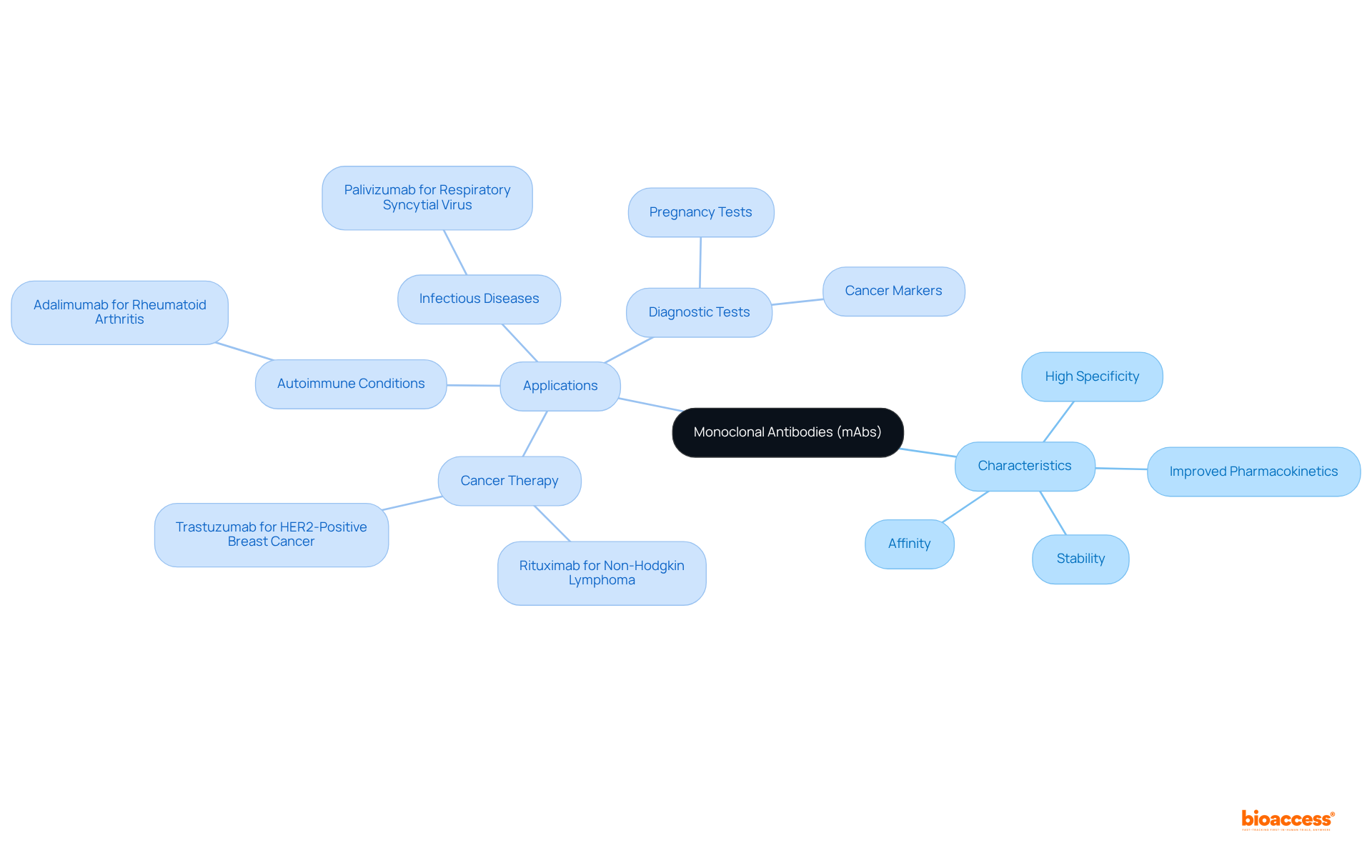
The characteristics of monoclonal antibodies, commonly represented by the mabs medical abbreviation, establish them as effective therapeutic agents. Their high specificity for target antigens significantly reduces off-target effects and enhances overall effectiveness. Moreover, these antibodies can be meticulously designed to improve pharmacokinetics, stability, and affinity for their targets.
The applications of monoclonal antibodies are extensive, spanning:
Additionally, monoclonal antibodies are utilized in diagnostic tests, including pregnancy tests and cancer markers, underscoring their versatility in both therapeutic and diagnostic arenas.
Monoclonal antibodies, commonly referred to as mabs, represent a pivotal advancement in medical science, significantly enhancing the capacity to diagnose and treat a diverse array of health conditions. Their engineered specificity facilitates targeted therapies, effectively minimizing side effects and establishing them as a cornerstone of modern medicine, particularly in oncology and immunology.
The significance of mabs spans various therapeutic areas, from cancer treatment to autoimmune disorders and infectious diseases. Noteworthy examples, such as trastuzumab for breast cancer and the role of monoclonal antibodies in combating COVID-19, underscore their transformative impact on patient outcomes. The historical context of mabs, beginning with their inception in the 1970s and culminating in the rapid development and approval of numerous therapies, illustrates the continuous evolution and expanding applications of these remarkable proteins.
Reflecting on the importance of mabs in healthcare highlights their potential to personalize treatment, tailoring therapies to individual patient needs. As research and technology advance, the role of monoclonal antibodies is poised to grow, promising even more innovative solutions in medical practice. Embracing this evolution not only enhances patient care but also underscores the critical importance of ongoing investment in biomedical research and development.
What does the abbreviation 'mabs' stand for in the medical field?
'Mabs' stands for monoclonal proteins, which are laboratory-engineered molecules that replicate the immune system's ability to combat harmful pathogens.
What is the purpose of monoclonal proteins?
Monoclonal proteins are designed to bind to specific antigens on cell surfaces, making them essential in diagnosing and managing various medical conditions.
What types of conditions can monoclonal proteins help diagnose and manage?
Monoclonal proteins are used in the diagnosis and management of cancers, autoimmune disorders, and infectious diseases.
How are monoclonal proteins produced?
Monoclonal proteins are derived from a single clone of a unique white blood cell, ensuring uniformity and targeting the same epitope, or specific segment of the antigen.