


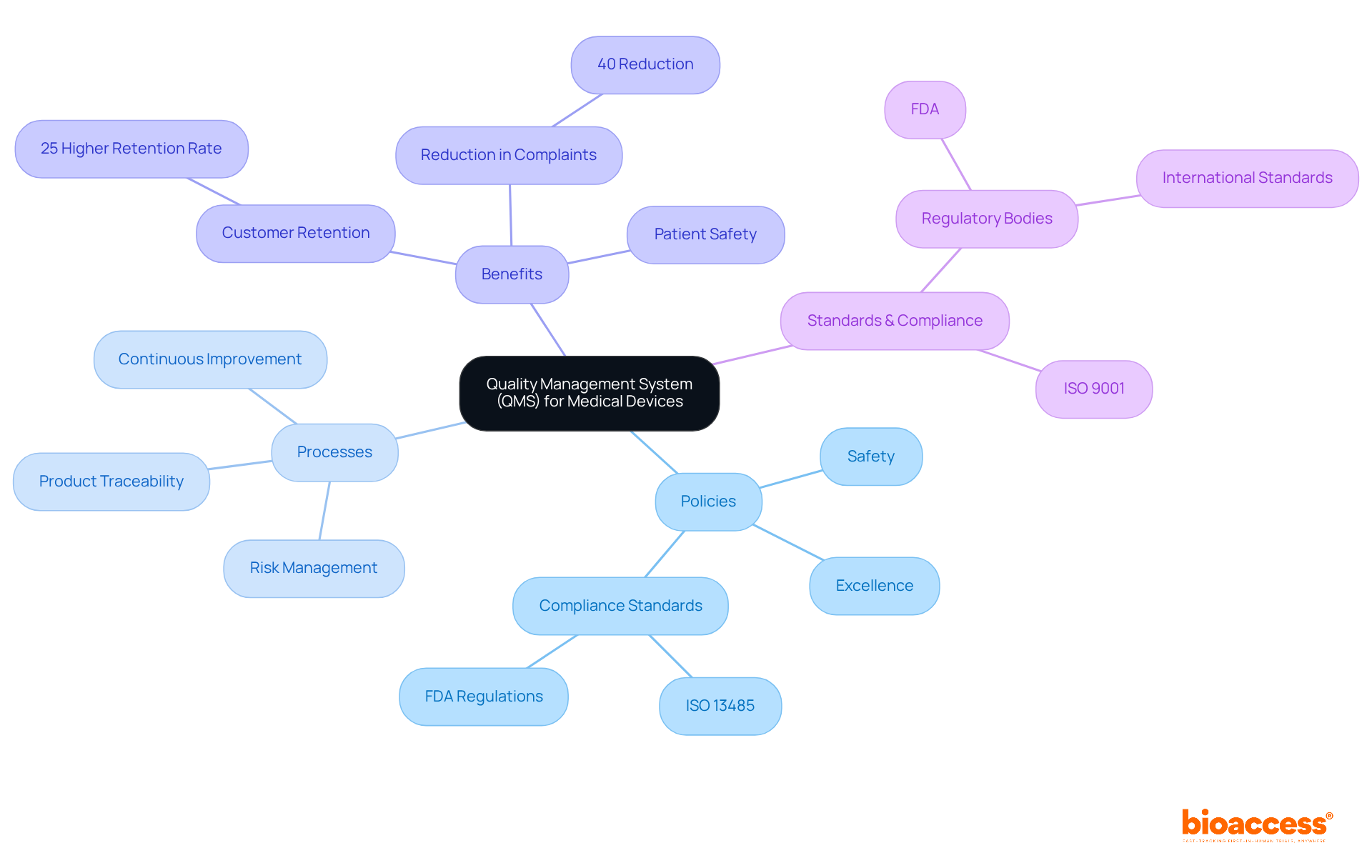
The article elucidates that a Quality Management System (QMS) for medical devices serves as a structured framework, ensuring that products consistently meet safety and compliance standards throughout their lifecycle. This adherence is critical for manufacturers to comply with regulations such as ISO 13485. Furthermore, it underscores that a robust QMS significantly enhances risk management, improves product traceability, and fosters continuous improvement. These factors ultimately lead to better patient safety and a reduction in customer complaints. Notably, statistics support the assertion that organizations implementing effective QMS practices experience substantial benefits.
A quality management system (QMS) for medical devices transcends mere regulatory compliance; it serves as a crucial framework that guarantees the safety and effectiveness of medical products throughout their lifecycle. By integrating essential policies and procedures, a robust QMS not only enhances risk management but also fosters continuous improvement, ultimately benefiting both manufacturers and patients. In light of evolving regulations and increasing market demands, organizations must consider:
A quality management system for medical devices serves as a structured framework that integrates policies, processes, and procedures designed to ensure that medical instruments consistently meet safety, excellence, and compliance standards throughout their lifecycle. This quality management system for medical devices is indispensable for manufacturers aiming to adhere to international standards such as ISO 13485 and regulatory bodies like the FDA.
A robust quality management system for medical devices not only enhances effective risk management and product traceability but also fosters continuous improvement, ultimately elevating patient safety and product reliability. Statistics indicate that organizations with a quality management system for medical devices achieve a 25% higher customer retention rate and a 40% reduction in customer complaints, underscoring the critical role of management in the medical equipment sector.
As William A. Foster aptly stated, 'Quality is never an accident; it is always the result of high intention, sincere effort, intelligent direction, and skillful execution.' This underscores the necessity of intentionality in establishing a successful QMS.
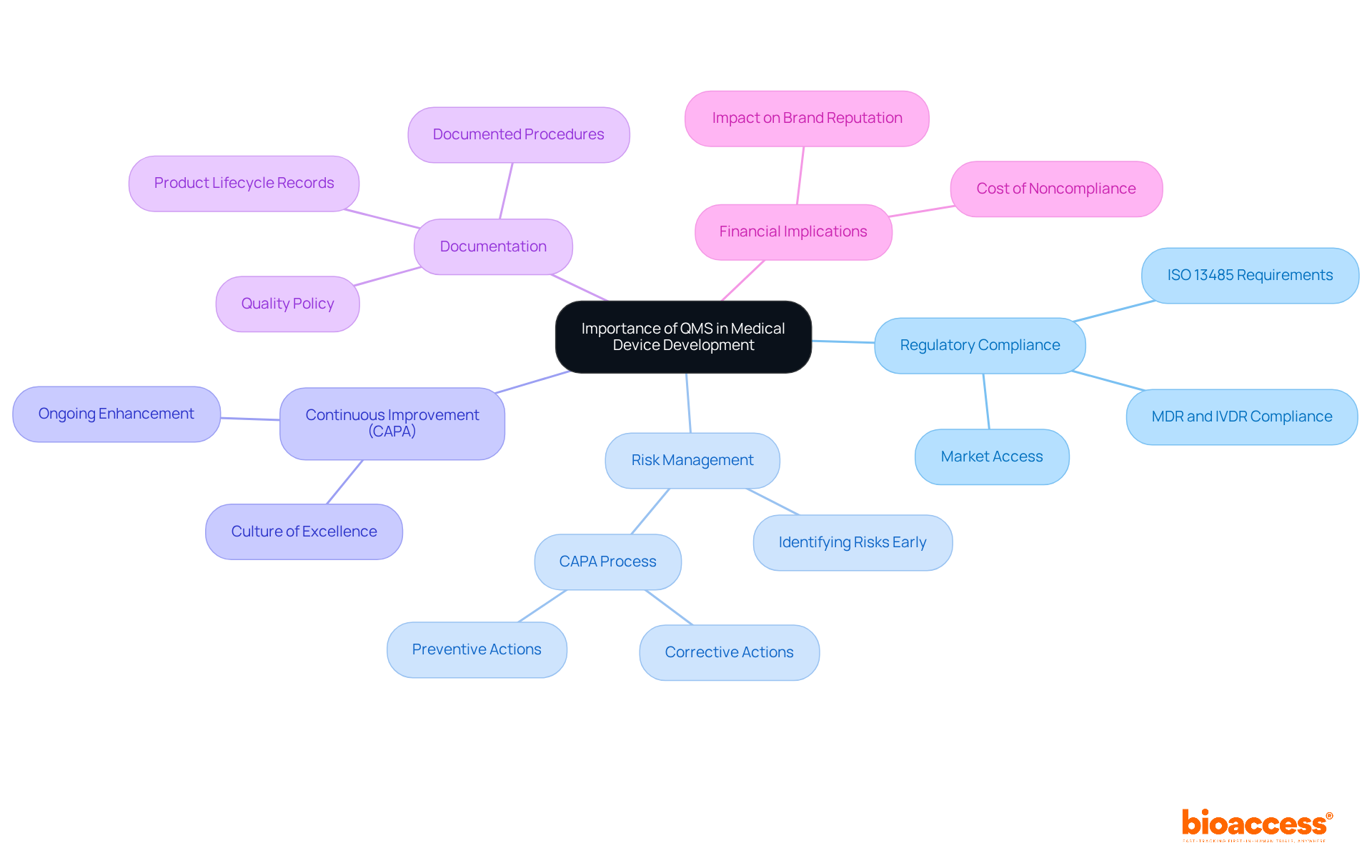
The significance of a quality management system for medical devices in the development process is paramount. It serves as the foundation of the manufacturing process, ensuring that products are designed, developed, and produced in accordance with strict quality standards, including the quality management system for medical devices requirements of the ISO 13485 standard, which is crucial for adherence to regulations. A well-executed QMS empowers organizations to recognize and tackle risks early in the development cycle, greatly reducing the likelihood of costly recalls and compliance penalties. Furthermore, producers of risk class I medical devices are now obligated to implement a quality management system for medical devices, emphasizing its essential role in the compliance framework.
The QMS encompasses the CAPA (Corrective and Preventive Actions) process, vital for addressing nonconformities and fostering continuous improvement. It cultivates a culture of excellence within the organization, encouraging ongoing enhancement and innovation. By adhering to a quality management system for medical devices, companies not only enhance their reputation and build trust with stakeholders but also contribute to improved patient outcomes. The financial consequences of ignoring management standards can be severe; inadequate practices can lead to higher expenses linked to recalls, compliance penalties, and damage to brand reputation.
Moreover, appropriate documentation and the creation of a quality policy are essential elements of a strong QMS, ensuring that all processes are well-defined and comply with legal requirements. Ultimately, a robust quality management system for medical devices is essential for ensuring the safety and effectiveness of medical products, aligning with both regulatory requirements and market expectations.
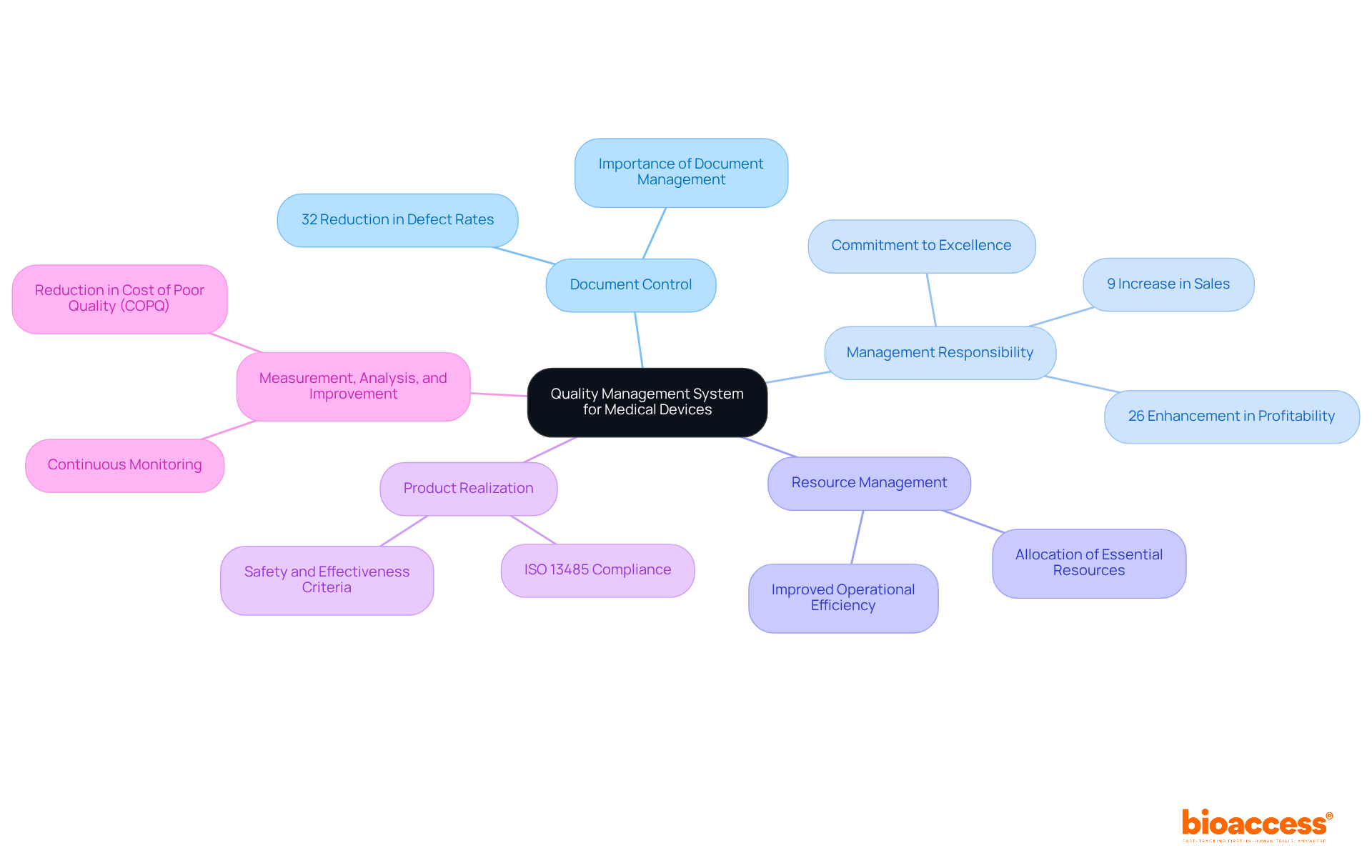
Key components of the quality management system medical devices are essential for ensuring compliance and enhancing product quality. These components include:
Document Control: This element ensures meticulous management, review, and updating of all documents related to standards processes. Effective document control is crucial; organizations with robust systems report a 32% reduction in defect rates, underscoring the importance of maintaining accurate and accessible documentation.
Management Responsibility: This component establishes the commitment of top management to excellence, clearly outlining their roles and responsibilities within the QMS. Studies indicate that organizations with strong management participation in improvement initiatives experience a 9% rise in sales and a 26% enhancement in profitability, emphasizing the strategic significance of leadership in fostering a culture focused on excellence.
Resource Management: Focused on the allocation of essential resources—personnel, infrastructure, and work environment—this aspect is vital for effective QMS implementation. Companies that emphasize resource management frequently experience improved operational efficiency and enhanced product standards, as sufficient resources are essential for adhering to compliance requirements.
Product Realization: This encompasses the entire process of bringing a medical device from concept to market, including design, development, and production. ISO 13485 mandates that organizations establish documented procedures for each stage, ensuring that products meet safety and effectiveness criteria throughout their lifecycle.
Measurement, Analysis, and Improvement: This involves continuous monitoring and measurement of QMS performance, analyzing data to identify areas for improvement, and implementing corrective actions. Companies that adopt a proactive approach to measurement and analysis can significantly reduce the Cost of Poor Quality (COPQ), which can range from 5% to 30% of sales revenue.
These components work synergistically to create a cohesive quality management system for medical devices that not only supports compliance with regulatory requirements but also enhances overall product quality, ultimately leading to greater customer satisfaction and retention.
A quality management system (QMS) for medical devices is essential for ensuring that products meet the highest standards of safety and effectiveness throughout their lifecycle. This structured framework aligns with international standards like ISO 13485 and plays a vital role in enhancing patient safety and product reliability. By integrating comprehensive policies and procedures, a robust QMS fosters a culture of continuous improvement, ultimately benefiting both manufacturers and consumers.
Key aspects of a QMS are highlighted, including:
These elements work together to create a cohesive system that ensures compliance with regulatory requirements while driving operational excellence and customer satisfaction. The statistics presented demonstrate the tangible benefits of implementing a QMS, such as reduced defect rates and improved profitability.
In conclusion, implementing a quality management system for medical devices is not merely a regulatory obligation; it is a strategic advantage that can significantly impact an organization’s success. As the medical device industry evolves, embracing current trends and best practices in quality management will be essential for fostering innovation and maintaining a competitive edge. Manufacturers are encouraged to prioritize the establishment and enhancement of their QMS to ensure they are compliant and positioned as leaders in quality and safety within the healthcare sector.
What is a Quality Management System (QMS) for medical devices?
A Quality Management System for medical devices is a structured framework that integrates policies, processes, and procedures to ensure that medical instruments consistently meet safety, quality, and compliance standards throughout their lifecycle.
Why is a QMS important for medical device manufacturers?
A QMS is crucial for manufacturers to adhere to international standards such as ISO 13485 and regulatory requirements from bodies like the FDA. It helps in effective risk management, product traceability, and fosters continuous improvement.
What are the benefits of having a robust QMS for medical devices?
Organizations with a robust QMS for medical devices experience a 25% higher customer retention rate and a 40% reduction in customer complaints, which highlights the importance of effective management in the medical equipment sector.
How does a QMS contribute to patient safety and product reliability?
By ensuring consistent adherence to safety and quality standards, a QMS enhances patient safety and product reliability, ultimately leading to better healthcare outcomes.
What does the quote by William A. Foster imply about quality?
The quote emphasizes that quality is the result of intentional effort, direction, and execution, highlighting the necessity of a deliberate approach in establishing a successful Quality Management System.