


Randomized Controlled Trials (RCTs) are pivotal in research, providing the highest standard of evidence for assessing the effectiveness and safety of new therapies. By minimizing bias through randomization and control groups, RCTs ensure robust findings.
Significantly, over 60% of new treatments receive approval based on RCT data, underscoring their critical role in shaping medical guidelines and healthcare policies. This influence reinforces the importance of RCTs in advancing medical knowledge and enhancing patient outcomes.
Randomized Controlled Trials (RCTs) are a cornerstone of clinical research, fundamentally transforming the evaluation of medical interventions for effectiveness and safety. By employing rigorous methodologies that minimize bias, RCTs provide a robust framework for testing new therapies and play a critical role in shaping healthcare policies and practices. As the complexity of medical research continues to evolve, researchers must consider how to ensure that these trials remain reliable and relevant in an ever-changing landscape.
Randomized Controlled Trials (RCTs) are a cornerstone of clinical research, as they are meticulously designed to minimize bias when assessing the effectiveness of new therapies or interventions, exemplifying the role of RCT in research. In a randomized controlled trial, participants are randomly allocated to either the experimental group, which receives the intervention, or the control group, which does not. This randomization is crucial; it ensures that any observed differences between the groups can be attributed directly to the treatment rather than confounding factors. Acknowledged as the gold standard in medical research, RCT in research provides robust evidence regarding the effectiveness and safety of interventions. In 2025, approximately 52% of clinical trials are classified as randomized controlled trials, underscoring their prevalence in the research landscape. The importance of randomization is further emphasized by experts who note that it balances treatment assignment across groups, thereby enhancing the validity of study outcomes. By effectively reducing bias, RCT in research plays a vital role in the advancement of medical knowledge and the improvement of patient care.
At bioaccess®, we deliver extensive management services for research studies, including:
Our expertise in conducting Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF) ensures that your studies are executed with precision and efficiency. We focus on comprehensive procedures such as experiment setup, ensuring adherence to regulatory standards, and efficient project management to enhance the success of your clinical studies. Ultimately, our efforts contribute to the advancement of medical technology and improved healthcare outcomes.

The concept of randomized controlled experiments (RCTs) has its roots in the early 20th century, significantly shaped by various researchers. A pivotal moment occurred in the 1940s when Sir Austin Bradford Hill conducted a groundbreaking study assessing the effectiveness of streptomycin in treating tuberculosis. This trial not only demonstrated the power of randomization in medical research but also set a standard for future methodologies.
The establishment of the CONSORT guidelines in the 1990s further solidified the standards for conducting randomized controlled trials, ensuring transparency and rigor in reporting. Since then, the number of randomized controlled trials has surged annually, highlighting the growing importance of RCT in research within the medical field.
Today, the role of RCT in research is characterized by increasingly complex designs that incorporate advanced statistical techniques and ethical considerations, reinforcing its foundational role in evidence-based medicine. Comprehensive research management services, such as those offered by bioaccess, are crucial for the effective execution of RCT in research. These services encompass:
This evolution illustrates the escalating complexity of clinical research and the necessity for robust support systems to drive the advancement of medical knowledge.
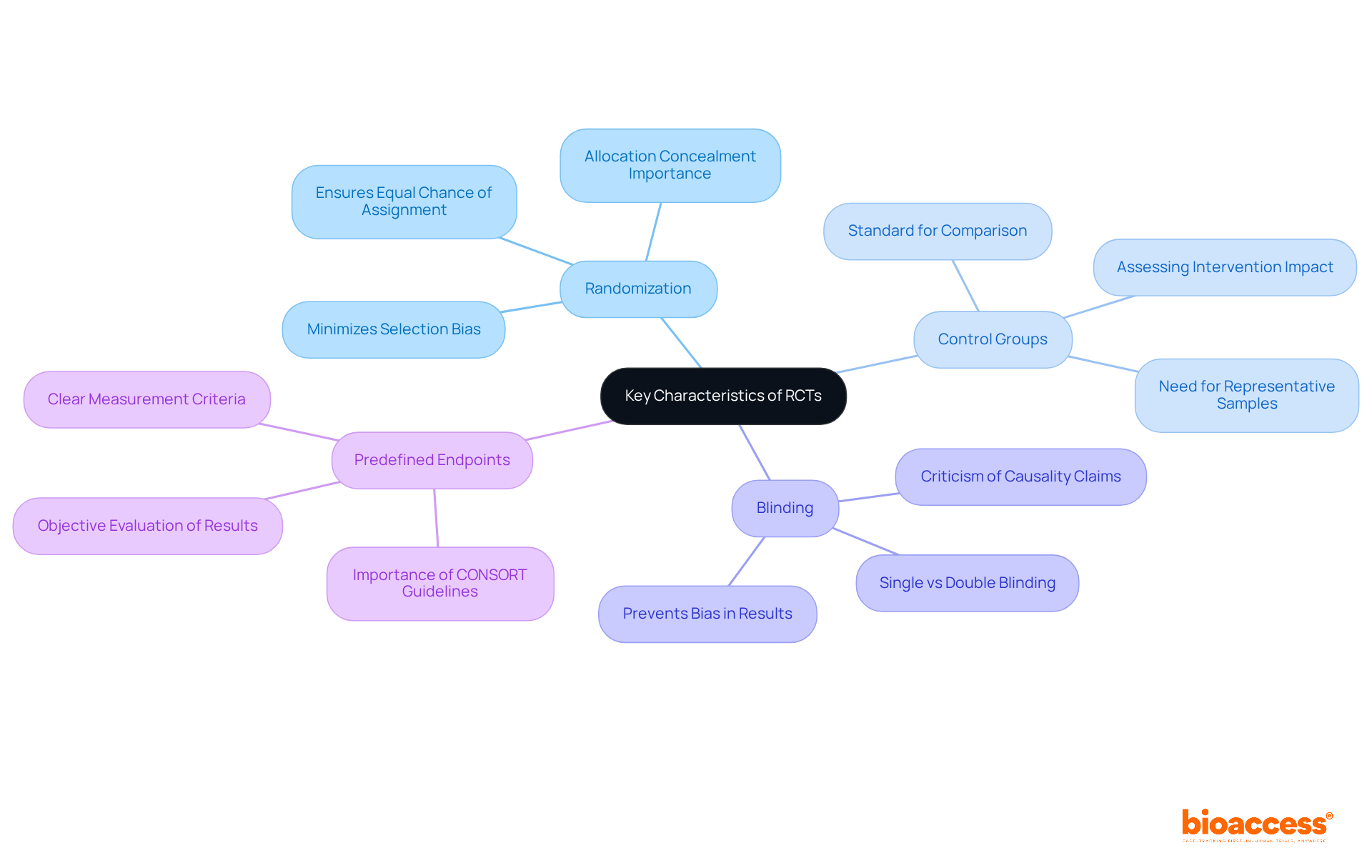
Key features of RCT in research include randomization, control groups, blinding, and predefined endpoints. Randomization is paramount, as it minimizes selection bias, ensuring that each participant has an equal chance of being assigned to any group. This is crucial for maintaining the integrity of the trial. A 2008 study concluded that randomized controlled trials with insufficient allocation concealment tended to be biased toward positive effects if outcomes were subjective, underscoring the significance of appropriate randomization techniques. Control groups serve as a standard, enabling researchers to assess the impacts of the intervention in relation to a group that does not receive it. This comparison offers a clearer comprehension of the intervention's effectiveness. For instance, RCTs of interventions reported a higher prevalence of comorbidities compared to vaccine trials, highlighting the need for representative samples in control groups.
Blinding, whether single or double, plays a significant role in preventing bias during intervention administration and outcome assessment. In double-blind studies, neither the participants nor the researchers know who is receiving the treatment, which helps eliminate expectations that could influence results. Dr. Steve Melia has criticized exaggerated assertions regarding randomized controlled trials in determining causality, suggesting that while they are valuable, their methodology must be scrutinized. This aspect of blinding is crucial for the dependability of randomized controlled trials, as it diminishes the likelihood of personal bias impacting the results.
Predefined endpoints are also critical, as they establish clear criteria for measuring the effectiveness of the intervention. By defining these endpoints in advance, researchers can objectively evaluate results, ensuring that the findings are based on predetermined metrics rather than post hoc analyses. The CONSORT guidelines emphasize the importance of clearly defined endpoints, which can enhance the validity of RCT findings. Collectively, these characteristics bolster the reliability and validity of RCT in research findings, thereby solidifying their status as a cornerstone of evidence-based medical research.
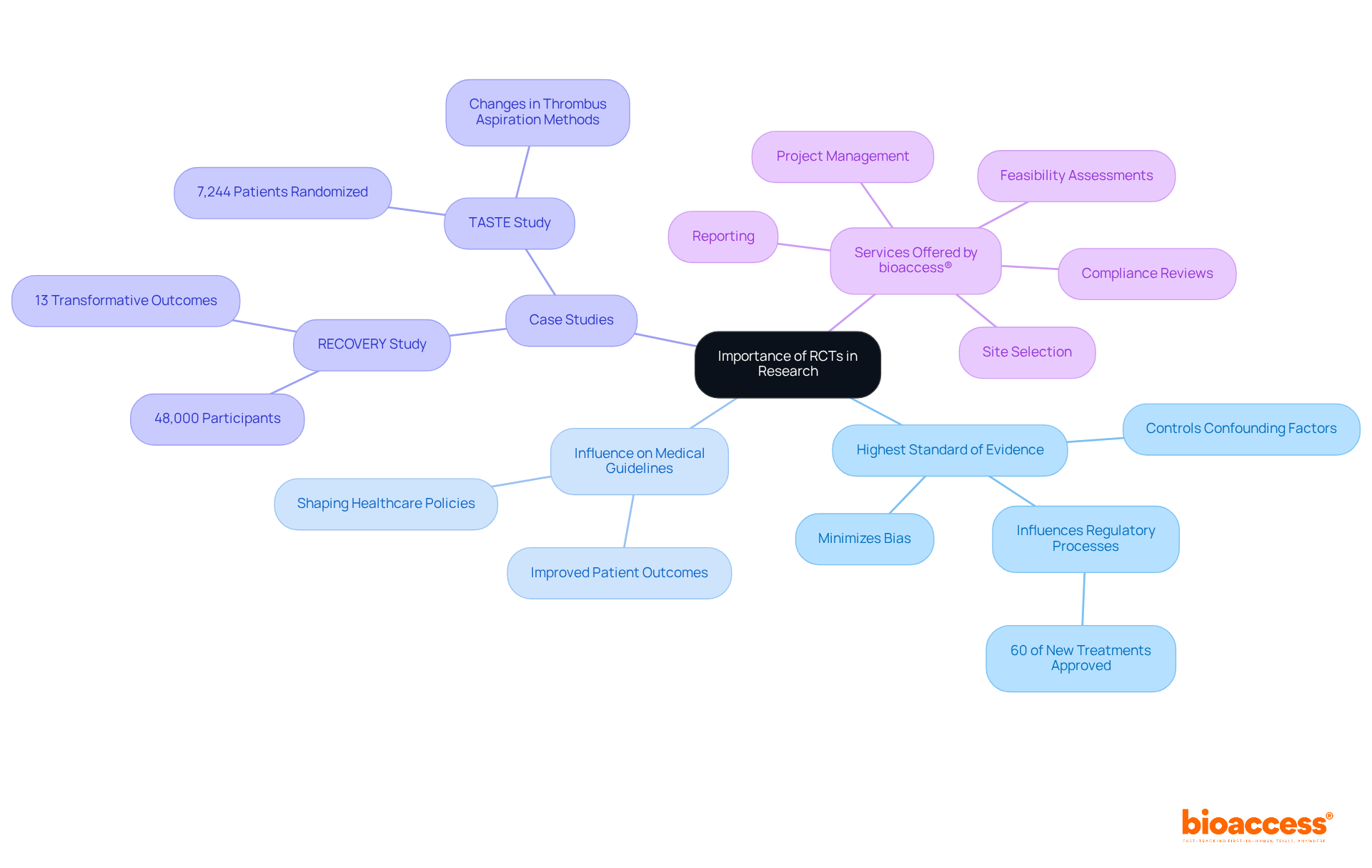
RCT in research are pivotal in medical studies, providing the highest standard of evidence for assessing the effectiveness and safety of new therapies. By minimizing bias and controlling for confounding factors, RCT in research enables researchers to draw accurate conclusions regarding intervention effects. The outcomes of these trials significantly influence medical guidelines and shape healthcare policies, ultimately leading to improved patient outcomes. Notably, over 60% of new treatments gain approval based on RCT data, highlighting their essential role in regulatory processes.
Case studies, such as the RECOVERY research, which involved over 48,000 participants and yielded 13 transformative outcomes, exemplify how randomized controlled studies can reshape clinical practices and guidelines. Furthermore, the TASTE study demonstrated the feasibility of conducting randomized controlled studies using existing healthcare data, resulting in crucial changes in thrombus aspiration methods.
Overall, RCT in research not only provides the necessary data to establish that a new treatment is effective and safe but also supports evidence-based healthcare practices.
At bioaccess®, we offer comprehensive study management services, encompassing:
Our expertise in managing various study categories—including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies—ensures that your research projects are executed efficiently and effectively, contributing to the advancement of medical research. Additionally, we adeptly navigate the regulatory landscape, including the role of INVIMA, to guarantee compliance with local regulations, thereby enhancing the integrity and success of your clinical trials.
Randomized Controlled Trials (RCTs) represent a cornerstone of clinical research, providing a robust framework for assessing the effectiveness and safety of new medical interventions. Their methodical design, marked by randomization, control groups, and blinding, mitigates bias and bolsters the reliability of outcomes, thus establishing RCTs as the gold standard in evidence-based medicine.
The historical progression of RCTs, from early experiments to the creation of CONSORT guidelines, highlights their increasing importance in medical research. Landmark studies, such as the pioneering streptomycin trial and the RECOVERY study, demonstrate how RCTs have not only influenced research methodologies but have also catalyzed significant advancements in clinical practice and healthcare policies. Furthermore, the integration of sophisticated statistical techniques and ethical considerations underscores the complexity and essential nature of RCTs in modern research.
Recognizing the significance of RCTs underscores their vital role in generating reliable data that shapes medical guidelines and regulatory approvals. As healthcare evolves, the dependence on meticulously designed RCTs becomes increasingly crucial for ensuring effective patient care. Engaging with the principles and practices of RCTs is imperative for researchers, practitioners, and policymakers alike, as it lays the groundwork for innovations that can profoundly enhance health outcomes and elevate the standards of medical practice.
What are Randomized Controlled Trials (RCTs)?
Randomized Controlled Trials (RCTs) are a type of clinical research designed to minimize bias when assessing the effectiveness of new therapies or interventions. In RCTs, participants are randomly assigned to either an experimental group that receives the intervention or a control group that does not.
Why is randomization important in RCTs?
Randomization is crucial in RCTs as it ensures that any observed differences between the groups can be attributed directly to the treatment rather than confounding factors. It balances treatment assignment across groups, enhancing the validity of study outcomes.
What is the significance of RCTs in medical research?
RCTs are acknowledged as the gold standard in medical research, providing robust evidence regarding the effectiveness and safety of interventions. They play a vital role in advancing medical knowledge and improving patient care.
What percentage of clinical trials were classified as RCTs in 2025?
In 2025, approximately 52% of clinical trials were classified as randomized controlled trials, highlighting their prevalence in the research landscape.
What management services does bioaccess® provide for research studies?
Bioaccess® offers extensive management services for research studies, including feasibility assessments, site selection, compliance reviews, setup, import permits, project management, and reporting.
What types of studies does bioaccess® conduct?
Bioaccess® conducts various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF).
How does bioaccess® ensure the success of clinical studies?
Bioaccess® focuses on comprehensive procedures such as experiment setup, adherence to regulatory standards, and efficient project management to enhance the success of clinical studies, contributing to the advancement of medical technology and improved healthcare outcomes.