


Regulatory considerations for vaccine trials in Serbia stand at a pivotal crossroads of legal, ethical, and scientific standards, ensuring the safety and efficacy of immunizations. This intersection is not just a bureaucratic necessity; it is essential for the integrity of clinical research. As the landscape of clinical trials evolves, grasping the frameworks established by the Medicines and Medical Devices Agency of Serbia (ALIMS) becomes crucial for researchers and organizations alike. However, navigating these complex regulations presents significant challenges.
How can stakeholders effectively align their practices with these stringent requirements while simultaneously fostering innovation in vaccine development?
Regulatory considerations for vaccine trials in Serbia are critical, encompassing the legal and ethical frameworks that govern clinical research involving immunizations. Compliance with national laws and adherence to Good Clinical Practice (GCP) guidelines are paramount, as well as the necessity of obtaining approvals from relevant regulatory bodies, which are crucial in the context of regulatory considerations for vaccine trials in Serbia. The Medicines and Medical Devices Agency of Serbia (ALIMS) plays a crucial role in supervising these studies, focusing on the regulatory considerations for vaccine trials in Serbia to ensure that they meet safety and efficacy standards before any vaccine can be administered to human subjects.
Local ethics committees typically conclude their assessments within 30 days, contributing to the efficiency of the regulatory process. Ethical considerations, such as informed consent and participant welfare, are essential to this framework, ensuring that experiments are conducted responsibly and ethically. Recent advancements have enabled some research study approvals to be finalized in as little as three weeks, reflecting the evolving landscape of medical research in Serbia. How does your organization navigate these regulatory challenges?
Furthermore, the role of local representatives as regulatory proxies is vital for compliance, facilitating communication with ALIMS and ensuring that all necessary documentation is submitted. To support these processes, bioaccess provides extensive clinical study management services, including:
This thorough approach underscores the commitment to upholding high ethical standards and participant rights throughout the study phases. Collaboration is key - what steps can you take to enhance your clinical research efforts?
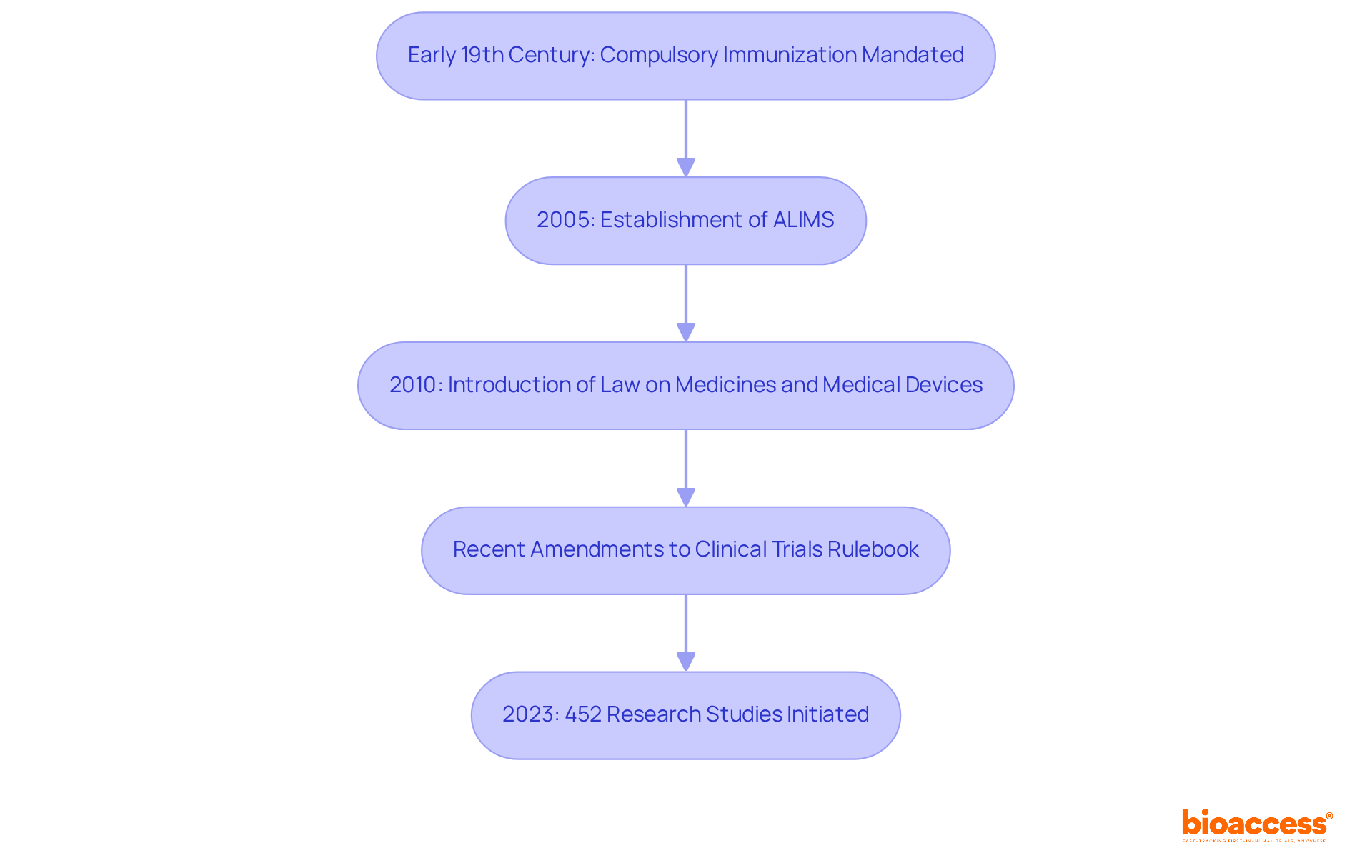
The historical background of inoculation research regulations in the region dates back to the early 19th century, when compulsory immunization was first mandated. This pivotal step initiated a regulatory evolution that has significantly transformed over the years. A crucial moment occurred in 2005 with the establishment of the Medicines and Medical Devices Agency (ALIMS), aimed at aligning the country's regulations with European Union standards. This alignment has been instrumental in enhancing the safety and effectiveness of clinical studies.
The introduction of the Law on Medicines and Medical Devices in 2010 further fortified this regulatory framework, ensuring that immunization studies are conducted under stringent guidelines that prioritize participant safety and ethical considerations. Recent amendments to the Clinical Trials Rulebook illustrate the country's commitment to adapting to global best practices and addressing emerging public health challenges. This reflects a proactive approach to vaccine regulation, which is essential when considering the regulatory considerations for vaccine trials in Serbia in today's rapidly evolving medical landscape.
Since 2023, the nation has initiated 452 research studies, showcasing the active involvement of the regulatory authority in promoting medical investigations. The evaluation procedure for research study approval typically requires 60 days, underscoring the effectiveness of the regulatory framework. As the country continues to develop its immunization regulations, the role of ALIMS remains crucial in overseeing adherence and ensuring the integrity of research studies, especially regarding the regulatory considerations for vaccine trials in Serbia.
In the country, the Medicines and Medical Devices Agency of the nation (ALIMS) serves as the primary regulatory authority overseeing the regulatory considerations for vaccine trials in Serbia. ALIMS meticulously assesses and authorizes clinical research applications, taking into account the regulatory considerations for vaccine trials in Serbia to ensure compliance with both national and international regulations. This diligence preserves the integrity of the clinical research process, particularly in the development of immunizations.
Complementing ALIMS, the Ethics Committee of Serbia rigorously reviews the ethical dimensions of proposed studies, placing a strong emphasis on participant welfare and informed consent. This collaboration fosters a robust regulatory environment that takes into account the regulatory considerations for vaccine trials in Serbia, ensuring safe and effective research on immunizations.
Recent updates from ALIMS reveal a streamlined process for vaccine study approvals, which incorporates regulatory considerations for vaccine trials in Serbia and reflects a commitment to enhancing research efficiency. In 2023, the country approved a significant number of clinical research applications, showcasing ALIMS's effectiveness in facilitating prompt research initiatives.
Local research institutions and ethics committees also play a crucial role in ensuring adherence to regulatory considerations for vaccine trials in Serbia, further bolstering the study process. This multi-faceted regulatory framework not only encourages compliance but also enhances the overall quality of medical studies conducted in the region, particularly regarding the regulatory considerations for vaccine trials in Serbia.
In this context, bioaccess® presents a unique advantage with its accelerated regulatory approval process, achieving approvals in just 6-8 weeks compared to the typical 6-12 months seen in the US and EU. This capability allows bioaccess® to enroll treatment-naive cardiology or neurology groups 50% faster than their Western counterparts, effectively addressing regulatory challenges and expediting the clinical research process.

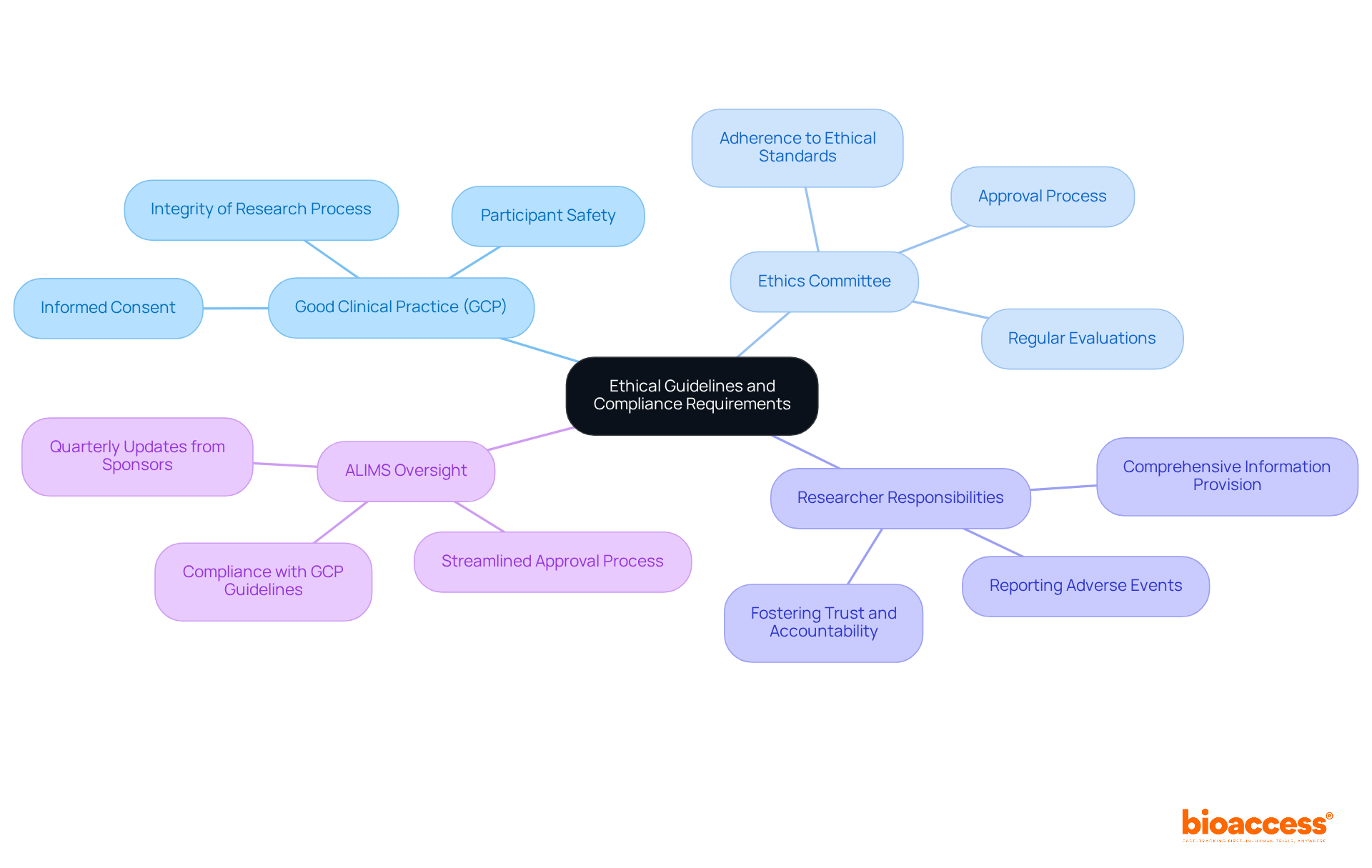
In Serbia, the regulatory considerations for vaccine trials in Serbia are grounded in Good Clinical Practice (GCP) standards, which prioritize participant safety, informed consent, and the integrity of the research process. Researchers must provide comprehensive information about the study's objectives, procedures, potential risks, and benefits before obtaining consent. This transparency is crucial for fostering trust and accountability in clinical research.
The Ethics Committee plays a vital role in this framework, conducting regular evaluations of ongoing studies to ensure adherence to ethical standards. Furthermore, researchers are obligated to promptly report any adverse events, reinforcing a culture of transparency throughout the study process. Such steadfast dedication to ethical adherence not only safeguards participants but also bolsters public confidence in the research initiatives undertaken in the region.
The Medicines and Medical Devices Agency (ALIMS) oversees compliance with GCP guidelines, ensuring that ethical standards are upheld across all research studies. Recent regulatory reforms have streamlined the approval process, emphasizing the regulatory considerations for vaccine trials in Serbia and positioning it as an increasingly attractive location for conducting such trials. With a growing number of ongoing studies, particularly in oncology, the commitment to ethical practices is essential for maintaining the integrity of research and enhancing Serbia's reputation as a hub for clinical studies.
The regulatory landscape for vaccine trials in Serbia is defined by a complex interplay of legal and ethical considerations, ensuring participant protection and research integrity. By adhering to national laws and Good Clinical Practice (GCP) guidelines, researchers and regulatory bodies like ALIMS create a safe environment for clinical investigations. This compliance is crucial for the successful development of vaccines.
Significant strides have been made in enhancing Serbia's regulatory framework, particularly through the establishment of ALIMS and the implementation of stringent ethical guidelines. The historical evolution of these regulations reflects a commitment to aligning with European standards, fostering a robust environment for clinical research. The collaboration between ALIMS, local ethics committees, and research institutions solidifies this framework, ensuring that vaccine trials meet safety and efficacy standards while prioritizing participant welfare.
As Serbia adapts to the dynamic field of medical research, it is essential for stakeholders to remain vigilant and proactive in navigating these regulatory considerations. Embracing transparency, ethical practices, and collaboration will enhance the quality of clinical studies and build public trust in vaccine research. Engaging with these regulatory frameworks is vital for advancing public health initiatives and ensuring Serbia remains a competitive player in the global landscape of vaccine development.
What are the main regulatory considerations for vaccine trials in Serbia?
The main regulatory considerations for vaccine trials in Serbia include compliance with national laws, adherence to Good Clinical Practice (GCP) guidelines, and obtaining approvals from relevant regulatory bodies, particularly the Medicines and Medical Devices Agency of Serbia (ALIMS).
What role does ALIMS play in vaccine trials?
ALIMS supervises vaccine trials in Serbia, ensuring that studies meet safety and efficacy standards before any vaccine can be administered to human subjects.
How quickly can local ethics committees assess vaccine trials?
Local ethics committees typically conclude their assessments within 30 days, contributing to the efficiency of the regulatory process.
What ethical considerations are important in vaccine trials?
Ethical considerations in vaccine trials include informed consent and participant welfare, ensuring that experiments are conducted responsibly and ethically.
How have recent advancements impacted the approval process for research studies in Serbia?
Recent advancements have enabled some research study approvals to be finalized in as little as three weeks, reflecting an evolving landscape of medical research in Serbia.
What is the role of local representatives in vaccine trials?
Local representatives act as regulatory proxies, facilitating communication with ALIMS and ensuring that all necessary documentation is submitted for compliance.
What clinical study management services does bioaccess provide to support vaccine trials?
Bioaccess provides extensive clinical study management services, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting.