


Early phase oncology trials are the cornerstone of developing new cancer therapies, especially in regions like Montenegro where regulatory frameworks are evolving. These trials not only evaluate the safety and efficacy of investigational drugs but also underscore the urgent need for innovative solutions in cancer treatment. However, the complex requirements and ethical considerations surrounding these trials can present significant challenges for researchers.
What strategies can be implemented to navigate these hurdles and ensure that promising therapies reach patients promptly?
Initial phase oncology studies, which encompass Phase I and Phase II investigations, are essential to fulfill the requirements for early phase oncology trials in Montenegro in the clinical assessment of new cancer therapies. These studies meticulously evaluate safety, tolerability, pharmacokinetics, and preliminary efficacy of investigational drugs in human subjects. Phase I studies typically involve a small group of individuals, focusing on establishing the maximum tolerated dosage and identifying side effects. In contrast, Phase II studies expand the participant pool to further assess treatment effectiveness and gather additional safety information.
Looking ahead to 2025, the anticipated number of early phase cancer research studies conducted globally signals a robust interest in innovative cancer treatments. Notably, there is expected to be a significant rise in study initiations targeting rare cancers. As oncologists emphasize, these trials are crucial for paving the way for later extensive studies, profoundly influencing the drug development process. The importance of Phase I and II oncology studies cannot be overstated; they lay the groundwork for advancements in cancer treatment, offering hope to patients and driving innovation in the field.
In this landscape, bioaccess® plays a pivotal role by providing comprehensive clinical study management services. These services include:
By adeptly navigating the complex requirements for early phase oncology trials in Montenegro and facilitating approval processes, bioaccess® accelerates the initiation of these critical studies. This ensures that innovative treatments reach patients more swiftly, reinforcing the collaborative efforts necessary for progress in clinical research.
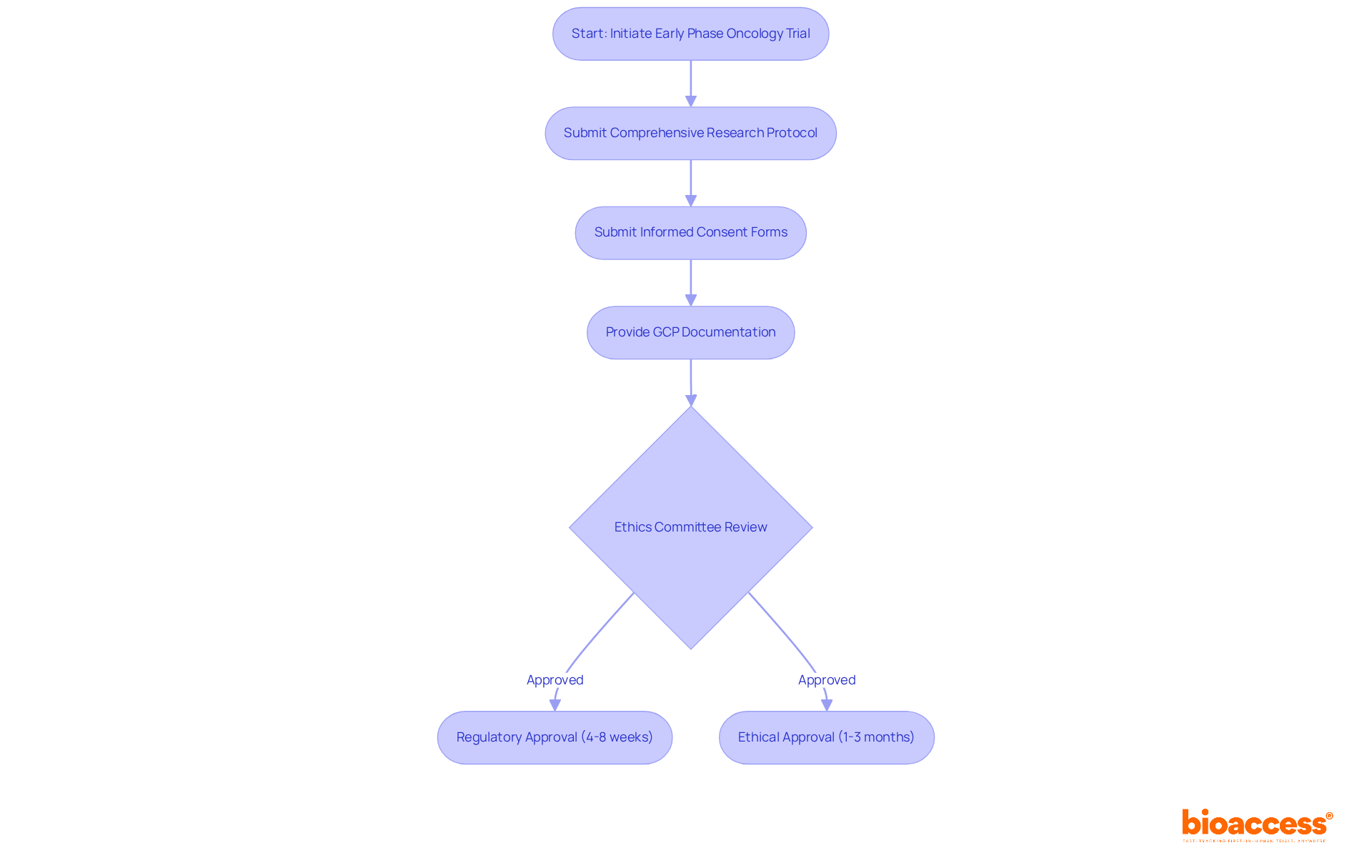
The regulatory framework governing early phase oncology studies in Montenegro is anchored by the Law on Medicines and the Law on Clinical Research, which outlines the requirements for early phase oncology trials in Montenegro. These laws mandate that all clinical studies, particularly those addressing the requirements for early phase oncology trials in Montenegro, secure approval from the Agency for Medicines and Medical Devices (CInMED). The requirements for early phase oncology trials in Montenegro include:
Furthermore, an independent ethics committee must conduct an ethical review to ensure the requirements for early phase oncology trials in Montenegro are met and to safeguard the rights and safety of participants.
The average time for regulatory approval in Montenegro typically spans from 4 to 8 weeks, while ethical approval may take an additional 1 to 3 months. This timeline positions Montenegro as an efficient option compared to other regions, making it an attractive destination for clinical research. With Bioaccess's expertise in regulatory approval, clinical research site activation, and patient recruitment, researchers can effectively navigate the requirements for early phase oncology trials in Montenegro. By utilizing their services, researchers can expedite site activation and ensure compliance, ultimately facilitating a quicker commencement of clinical studies.
In a landscape where timely approvals are crucial, the collaboration with Bioaccess not only streamlines the process but also enhances the overall efficiency of clinical research initiatives. As you consider your own challenges in clinical research, think about how partnering with a knowledgeable ally like Bioaccess can make a significant difference.
The requirements for early phase oncology trials in Montenegro include ethical factors that are crucial for safeguarding the rights and well-being of participants. Informed consent stands as a foundational element, necessitating that individuals are comprehensively informed about the trial's objectives, procedures, potential risks, and benefits before agreeing to participate. This process transcends mere formality; it is vital for fostering trust and transparency between researchers and subjects. Bioethicists assert that informed consent should evolve into an ongoing dialogue, enabling participants to pose questions and withdraw at any time without facing penalties.
The role of ethical review boards is indispensable in this context. These boards meticulously evaluate the ethical implications of the study, ensuring compliance with established guidelines, such as the Declaration of Helsinki. They scrutinize the study design to mitigate risks while maximizing potential benefits, a necessity given that phase I studies frequently involve novel therapies with uncertain safety profiles. Recent data reveal that approval rates from ethical review boards for studies meeting the requirements for early phase oncology trials in Montenegro can vary significantly, reflecting the intricate and sensitive nature of the research involved.
Moreover, continuous oversight of participants' well-being throughout the study is essential. This ongoing monitoring facilitates the prompt identification and resolution of any emerging ethical concerns, ensuring that participant welfare remains paramount. As the landscape of clinical research evolves, a steadfast commitment to ethical standards and informed consent procedures will be vital in preserving the integrity of cancer studies.
Carrying out initial stage cancer studies requires addressing the requirements for early phase oncology trials in Montenegro, which presents significant challenges, particularly in patient recruitment, regulatory compliance, and logistical coordination. These hurdles are not just procedural; they directly impact the success of clinical trials and the advancement of oncology research. For instance, strict eligibility criteria often limit the pool of potential candidates, leading to dropout rates in early phase oncology studies that can soar as high as 20%. This statistic underscores the critical need for maintaining participant engagement throughout the research process.
Efficient communication and support are essential in addressing these dropout rates. Many patients express concerns about adverse events and the complexity of research protocols, which can deter their participation. Therefore, clinical study managers must prioritize customized patient recruitment strategies. Utilizing digital tools and social media can significantly enhance outreach, particularly among younger demographics who are more engaged online. Moreover, providing logistical support, such as home visits for check-ins, can greatly improve participation rates, especially for patients with chronic diseases facing mobility challenges.
The requirements for early phase oncology trials in Montenegro add another layer of complexity to these studies. Researchers must meticulously prepare and submit documentation that adheres to the requirements for early phase oncology trials in Montenegro, a process that can be daunting for those unfamiliar with specific regulations in regions like Montenegro and North Macedonia. Additionally, logistical challenges, such as coordinating various locations and managing materials, can complicate execution.
To navigate these complexities effectively, fostering open communication is vital. By implementing tailored strategies and ensuring that patients feel supported, researchers can better manage the intricacies of early phase oncology trials. This approach not only enhances participant retention but also contributes to improved overall outcomes in clinical research.
The landscape of early phase oncology trials in Montenegro is characterized by a structured approach to evaluating innovative cancer therapies through rigorous Phase I and II studies. These trials are essential for establishing the safety and effectiveness of new treatments, ultimately shaping the future of oncology and offering hope to patients battling cancer. Collaborative efforts from organizations like bioaccess® play a crucial role in navigating the complex regulatory and ethical requirements, ensuring that these vital studies can proceed efficiently.
Key insights from the article underscore the significance of:
The necessity for informed consent, adherence to Good Clinical Practice, and the active involvement of ethics committees are pivotal in safeguarding participants and enhancing the integrity of the research process. Moreover, addressing recruitment challenges and logistical hurdles is critical for the success of these trials, as they directly impact patient engagement and study outcomes.
In summary, the ongoing commitment to advancing early phase oncology trials in Montenegro is vital for the future of cancer treatment. Stakeholders within the clinical research community must prioritize:
to overcome existing barriers and improve the efficiency of trial execution. By fostering an environment conducive to testing and refining groundbreaking therapies, the potential for significant advancements in cancer care becomes increasingly attainable.
What are early phase oncology trials?
Early phase oncology trials, which include Phase I and Phase II studies, are initial investigations that evaluate the safety, tolerability, pharmacokinetics, and preliminary efficacy of new cancer therapies in human subjects.
What is the focus of Phase I studies?
Phase I studies typically involve a small group of individuals and focus on establishing the maximum tolerated dosage of a drug and identifying its side effects.
How do Phase II studies differ from Phase I studies?
Phase II studies expand the participant pool to further assess the effectiveness of the treatment and gather additional safety information, building on the findings from Phase I.
What is the significance of early phase oncology trials for cancer treatment?
These trials are crucial for paving the way for later extensive studies, significantly influencing the drug development process and offering hope to patients by driving innovation in cancer treatment.
What is the expected trend for early phase cancer research studies by 2025?
There is an anticipated significant rise in the number of early phase cancer research studies globally, particularly targeting rare cancers, indicating a robust interest in innovative cancer treatments.
What role does bioaccess® play in early phase oncology trials?
Bioaccess® provides comprehensive clinical study management services, including feasibility assessments, site selection, compliance evaluations, study setup, and project management, to facilitate and accelerate the initiation of early phase oncology trials in Montenegro.
How does bioaccess® contribute to the approval processes for oncology trials?
By navigating the complex requirements for early phase oncology trials and facilitating approval processes, bioaccess® ensures that innovative treatments reach patients more swiftly, enhancing progress in clinical research.