


The fragment crystallizable region (Fc region) in antibodies plays a pivotal role in mediating immune responses through its interaction with Fc receptors on immune cells. This interaction facilitates critical processes such as:
The structural importance of the Fc region, along with its therapeutic implications, cannot be overstated. Modifications made to the Fc region can significantly enhance the efficacy of monoclonal antibodies, improving their interactions with immune components. Ultimately, these advancements lead to better clinical outcomes, underscoring the necessity of ongoing research and innovation in this field.
The fragment crystallizable region, or Fc region, represents a pivotal element of antibodies, acting as a vital link between the immune system and its defense mechanisms. Grasping the structure and function of the Fc region not only clarifies its role in immune responses but also unveils its transformative potential in therapeutic applications.
How might modifications to this small yet powerful segment enhance the efficacy of treatments for complex diseases such as cancer and autoimmune disorders? Delving into this inquiry reveals the intriguing intersection of immunology and innovative medicine.
The fragment crystallizable region, commonly referred to as the Fc portion, represents the tail end of an antibody molecule. This segment, known as the fragment crystallizable region, comprises the constant regions of the heavy chains and is vital for facilitating interactions with various defense components and proteins. Notably, the fragment crystallizable region of the Fc part does not directly attach to antigens; instead, it engages with Fc receptors on the surface of defense cells, promoting essential responses such as opsonization and complement activation. This area, particularly the fragment crystallizable region, is instrumental in defining the effector roles of the immune molecule, significantly influencing how the body's defense system responds to pathogens and other external substances.
Research underscores the importance of the fragment crystallizable region, revealing that antibodies with enhanced Fc regions can amplify antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP), both of which are critical for effective defense responses. Moreover, the half-life of immune proteins, such as IgG1 and IgG2, can extend to 21 days, illustrating the fragment crystallizable region's role in sustaining immune responses over time. Recent advancements in Fc engineering have demonstrated that strategic modifications to the fragment crystallizable region can improve therapeutic efficacy, highlighting its pivotal role in immunoglobulin function and its impact on clinical outcomes.
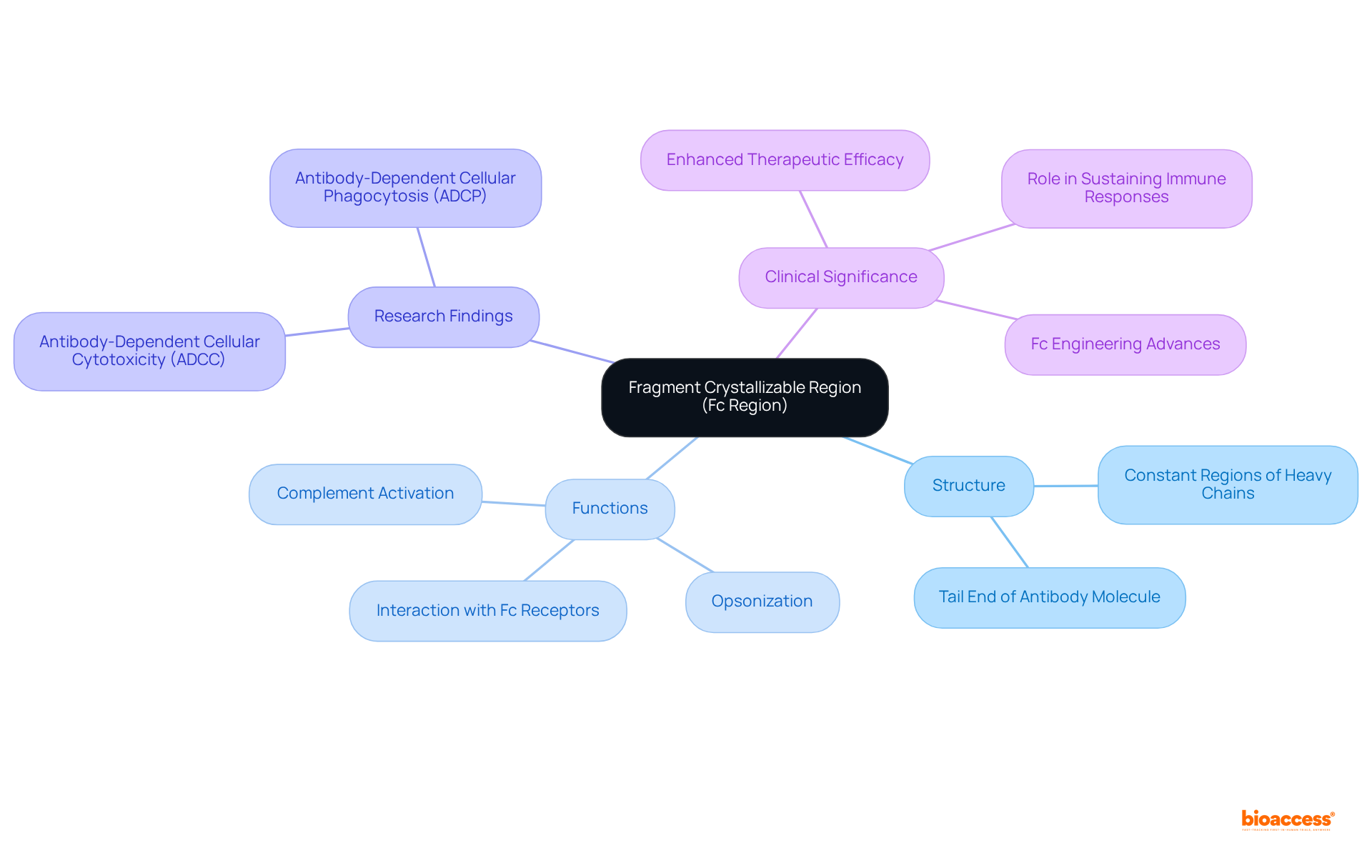
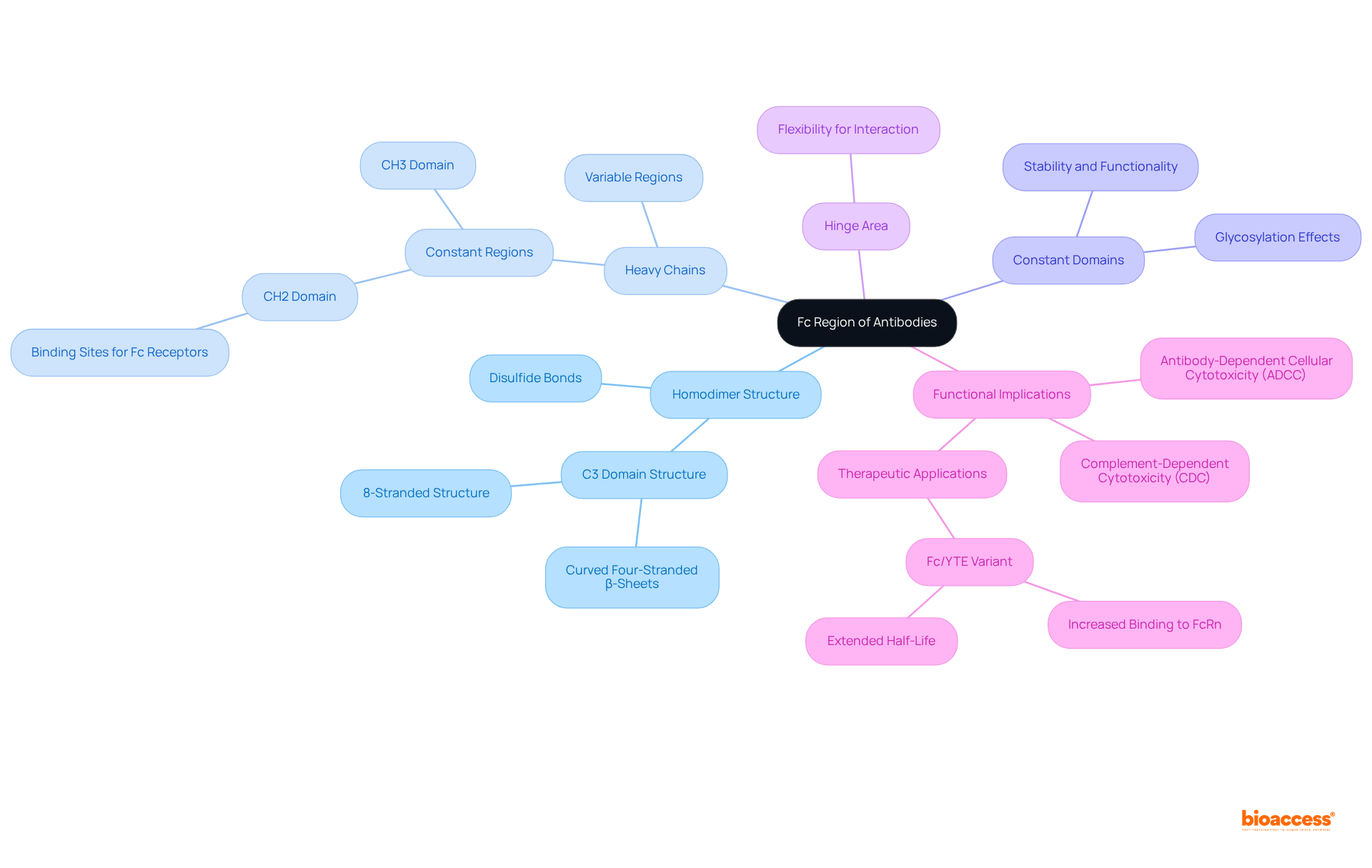
The Fc portion of antibodies is fundamentally organized as a homodimer, comprising two identical heavy chains linked by disulfide bonds. Each heavy chain typically features two to three constant domains, specifically CH2 and CH3, which are crucial for the stability and functionality of the Fc region. Notably, the CH2 domain is essential, as it houses the binding sites for Fc receptors, facilitating interactions with defense cells. The hinge area, positioned between the Fab and Fc sections, provides the necessary flexibility for optimal placement of the Fc section, thereby enhancing its interaction with effector cells. This structural configuration is vital for the Fc region’s capacity to mediate various immune responses, including antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).
Recent studies underscore that the dimerization of the Fc predominantly occurs through the C3 domain's curved four-stranded antiparallel β-sheets, which culminate in an intricate 8-stranded structure. Furthermore, the half-life of IgG is approximately 21 days, underscoring the significance of the Fc portion in therapeutic applications. The Fc/YTE variant demonstrates an approximately 8-fold increase in binding to human FcRn at pH 6.0, illustrating the structural implications of the Fc portion and its interactions. Understanding these structural nuances is essential for advancing therapeutic protein design and enhancing their efficacy in clinical applications.
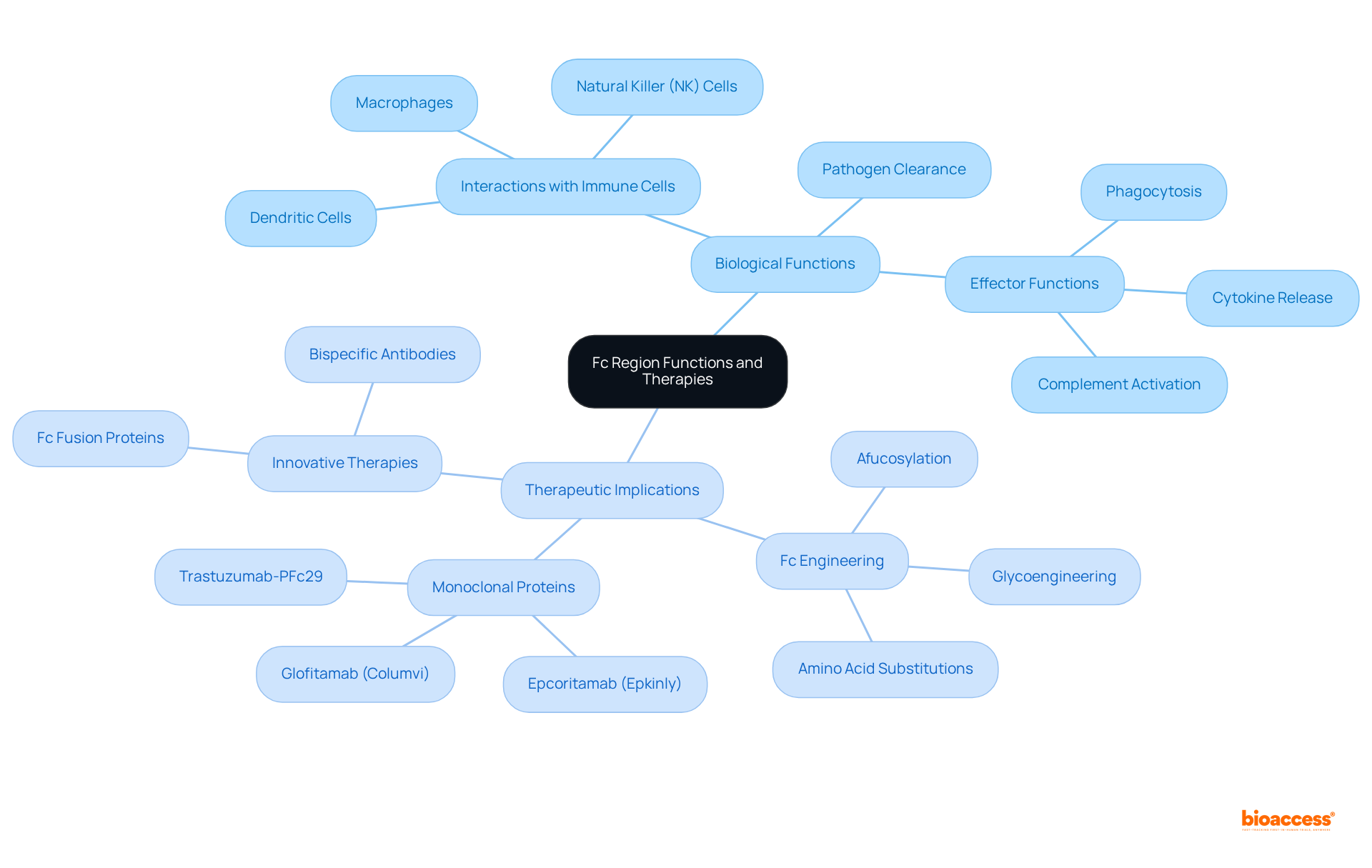
The Fc part plays a pivotal role in coordinating defense reactions by facilitating interactions with Fc receptors on defense units, including macrophages, natural killer (NK) units, and dendritic units. These interactions are critical for triggering effector functions such as phagocytosis, cytokine release, and complement activation, all essential for effective pathogen clearance.
In therapeutic contexts, modifications to the fragment crystallizable region significantly enhance the effectiveness of monoclonal proteins by improving their interactions with immune components and extending their serum half-life. For instance, afucosylated immunoglobulins exhibit increased antibody-dependent cellular cytotoxicity (ADCC) due to enhanced binding to FcγRIIIA, the predominant activating Fc receptor on NK cells.
Recent advancements in Fc engineering, especially in the fragment crystallizable region, have ushered in innovative therapies, including bispecific molecules and Fc fusion proteins, which expand the therapeutic landscape for conditions such as cancer and autoimmune disorders. These engineered antibodies not only enhance clinical outcomes but also tackle the challenges posed by traditional therapies, underscoring the transformative potential of Fc modifications in modern medicine.
The fragment crystallizable region, or Fc region, is an essential component of antibodies, effectively linking the immune system's response capabilities with advancements in therapy. Understanding its structure and function not only elucidates how it mediates interactions with immune cells but also underscores its pivotal role in enhancing treatment efficacy for a range of diseases.
Key insights from the article emphasize the Fc region's critical function in facilitating immune responses through its interactions with Fc receptors on defense cells. The structural intricacies of the Fc portion, including its dimeric nature and the significance of specific domains, are vital for mediating functions such as antibody-dependent cellular cytotoxicity (ADCC) and complement activation. Moreover, recent developments in Fc engineering illustrate how modifications can optimize therapeutic outcomes, particularly concerning monoclonal antibodies and innovative treatments for conditions like cancer and autoimmune disorders.
Reflecting on the transformative potential of the Fc region highlights its importance not only in immunology but also in modern medicine. As research continues to reveal new applications and enhancements, the Fc region emerges as a focal point for future therapeutic strategies, inviting further exploration and innovation in the pursuit of effective treatments. By acknowledging the critical role of the Fc region, stakeholders in healthcare and research can leverage its capabilities to improve patient outcomes and tackle complex medical challenges.
What is the fragment crystallizable region (Fc region) of an antibody?
The fragment crystallizable region, or Fc region, is the tail end of an antibody molecule that comprises the constant regions of the heavy chains. It is crucial for interactions with various defense components and proteins.
How does the Fc region interact with the immune system?
The Fc region does not directly bind to antigens; instead, it interacts with Fc receptors on the surface of defense cells, promoting responses such as opsonization and complement activation.
What roles does the Fc region play in the immune response?
The Fc region is instrumental in defining the effector roles of antibodies, significantly influencing the body's defense system's responses to pathogens and external substances.
How can modifications to the Fc region enhance immune responses?
Research shows that antibodies with enhanced Fc regions can amplify antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP), which are critical for effective defense responses.
What is the significance of the half-life of immune proteins related to the Fc region?
The half-life of immune proteins like IgG1 and IgG2 can extend up to 21 days, indicating the Fc region's role in sustaining immune responses over time.
What advancements have been made in Fc engineering?
Recent advancements in Fc engineering have shown that strategic modifications to the Fc region can improve therapeutic efficacy, highlighting its importance in immunoglobulin function and clinical outcomes.