


Understanding the investigational drug importation protocol in Romania reveals a complex yet vital framework that governs the entry of new medicinal products for clinical research. This protocol not only ensures compliance with stringent safety and efficacy standards but also positions Romania as an emerging hub for innovative medical studies.
However, as the landscape of clinical trials evolves, what challenges and opportunities arise from navigating these regulatory waters? Exploring these questions sheds light on the implications for researchers and pharmaceutical companies alike.
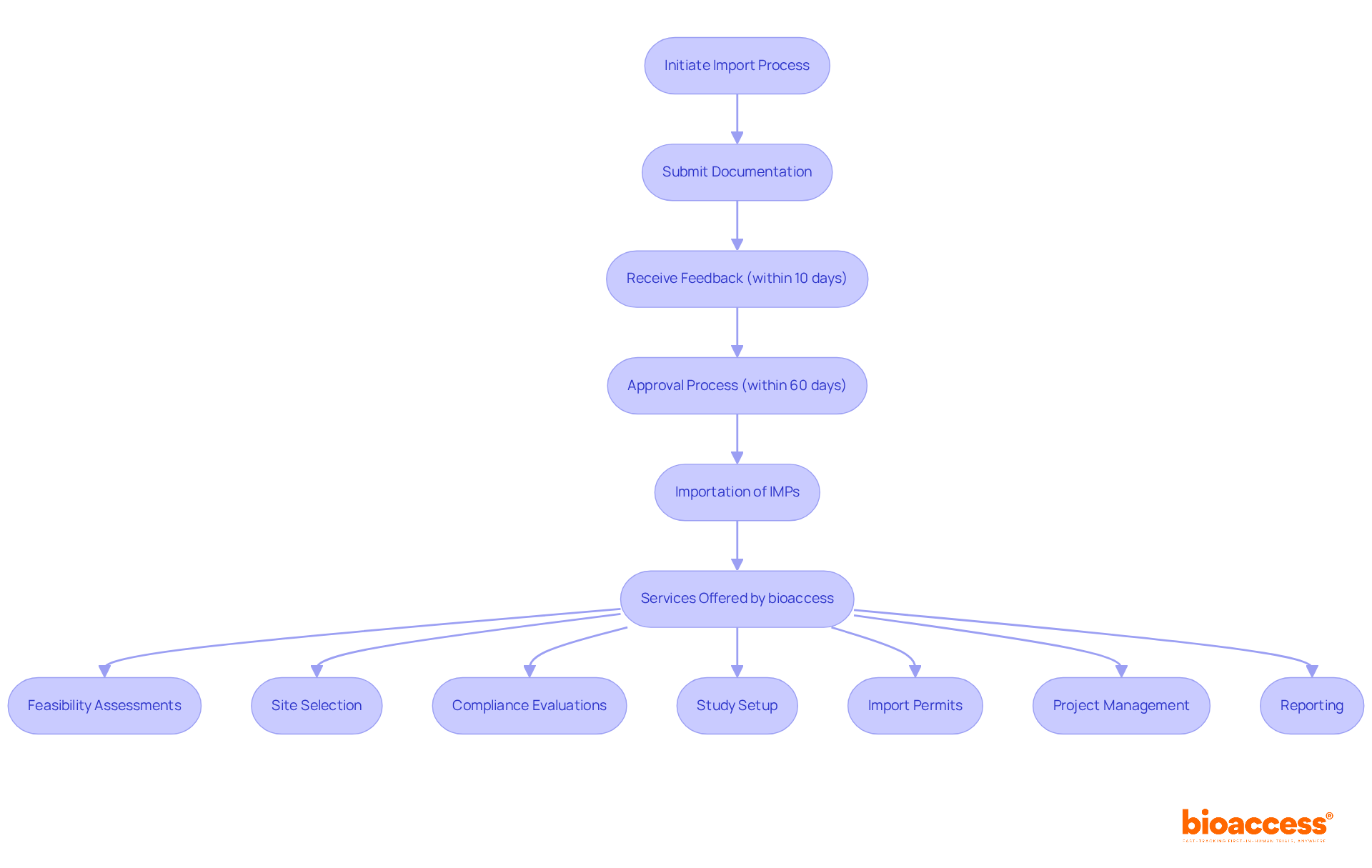
The investigational drug importation protocol in Romania establishes a crucial regulatory framework for importing investigational medicinal products (IMPs) intended for research trials. This protocol is vital for ensuring that all imported drugs meet stringent safety and efficacy standards, aligning with both national and European Union regulations. It outlines the necessary documentation, approvals, and procedures for the investigational drug importation protocol in Romania, promoting ethical and responsible use in experimental settings.
The investigational drug importation protocol in Romania, overseen by the National Agency for Medicines and Medical Devices (NAMMD), plays a pivotal role in authorizing the importation process and ensuring compliance with Good Clinical Practice (GCP) guidelines. This organized approach not only facilitates the seamless import of IMPs but also reinforces the nation's commitment to maintaining high standards in medical studies. Furthermore, bioaccess offers extensive research study management services, including:
These services are essential for navigating the complexities of the regulatory landscape and ensuring adherence to the investigational drug importation protocol in Romania.
The country presents significant advantages for conducting medical studies, such as high patient availability and expedited recruitment timelines, both critical for the success of medical investigations. Currently, there are 2,618 studies registered, with 265 actively recruiting investigations, underscoring the nation's growing importance in the medical experimentation landscape. Moreover, the regulatory body provides a structured schedule for research approvals, ensuring that applicants receive feedback on their submissions within 10 days and authorization within a maximum of 60 days. This efficiency enables the prompt commencement of studies, fostering an environment conducive to innovative research.
The investigational drug importation protocol in Romania plays a crucial role in medical studies by regulating the entry of new therapeutic products into the country for testing. The investigational drug importation protocol in Romania not only ensures the secure importation of investigational drugs but also accelerates the progress of research studies, which are vital for assessing the safety and effectiveness of new treatments. By streamlining the investigational drug importation protocol in Romania, the country enhances its reputation as a burgeoning hub for medical studies, attracting global pharmaceutical firms and fostering innovation within the healthcare sector.
Adhering to the investigational drug importation protocol in Romania is essential for maintaining the integrity of medical studies and safeguarding patient safety, thereby boosting public confidence in the investigative process. Specialist perspectives highlight that the transparency and efficiency of the investigational drug importation protocol in Romania significantly impact the speed at which medical studies can commence, making the country an attractive destination for pharmaceutical firms looking to conduct investigations. Case studies reveal that compliance with the investigational drug importation protocol in Romania has resulted in successful trial outcomes, underscoring the effectiveness of this protocol in facilitating timely access to investigational products.
As the country continues to advance in the field of medical inquiry, the investigational drug importation protocol in Romania remains a cornerstone for ensuring that innovative therapies reach patients safely and efficiently. This commitment not only enhances the research landscape but also reinforces the importance of collaboration among stakeholders to address key challenges in clinical research.
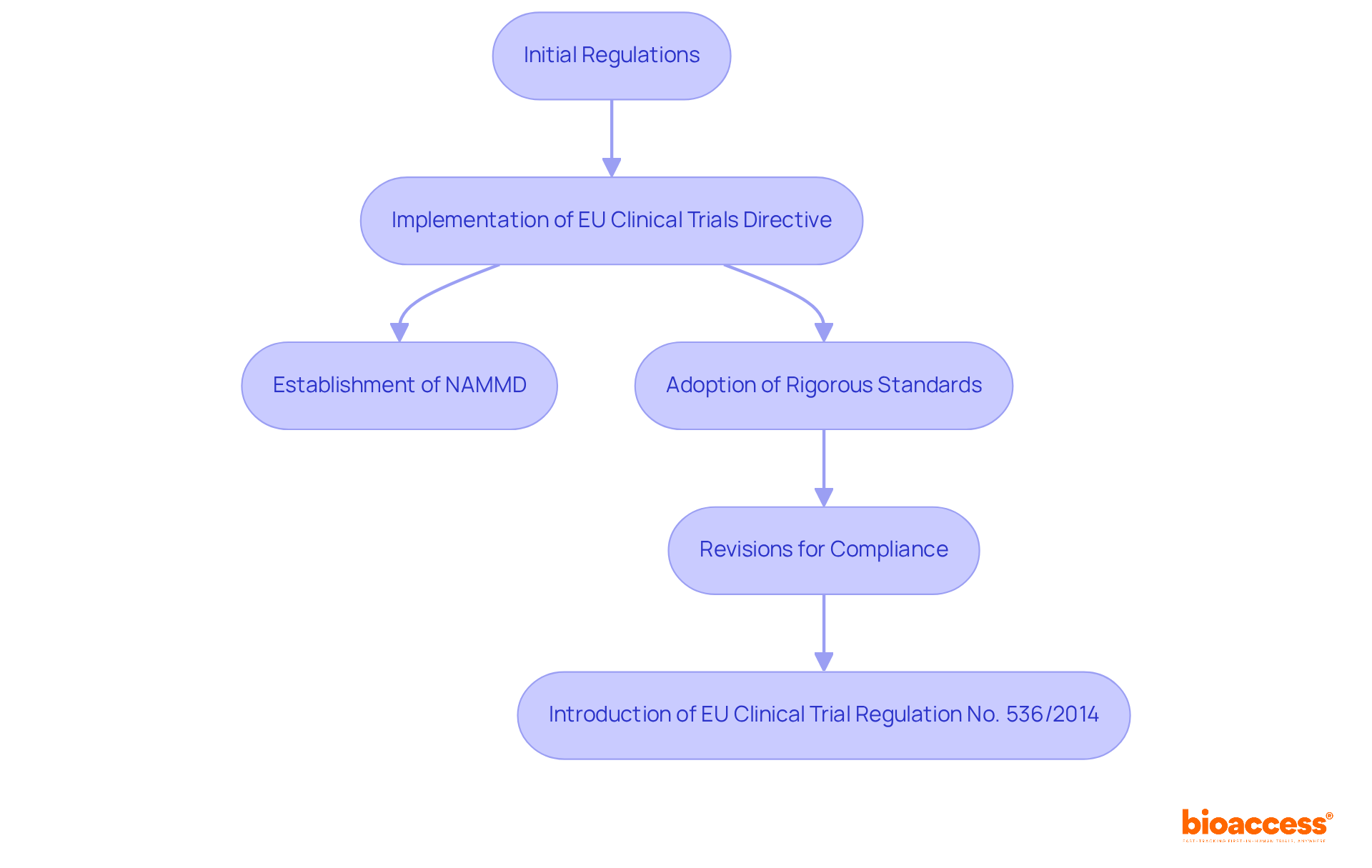
The historical evolution of the investigational drug importation protocol in Romania is intricately linked to the country's integration into the European Union, necessitating the alignment of its regulatory framework with EU standards. Initially, the importation of investigational drugs faced less stringent regulations, which posed challenges in ensuring the safety and efficacy of these products. However, the implementation of the EU Clinical Trials Directive marked a pivotal moment, leading to the establishment of the National Agency for Medicines and Medical Devices (NAMMD) and the adoption of more rigorous standards for clinical trials.
Over the years, the investigational drug importation protocol in Romania has undergone multiple revisions aimed at enhancing compliance with international best practices, streamlining processes, and addressing emerging challenges within the pharmaceutical landscape. Notably, the new EU Clinical Trial Regulation No. 536/2014 has further simplified the application process, allowing for a single submission to the Regulatory Authority and Ethics Committee across all involved EU countries. This evolution underscores the country's commitment to fostering a robust medical investigation environment while prioritizing patient safety and ethical standards.
Moreover, bioaccess plays a crucial role in this landscape by providing comprehensive study management services. These include:
The research market in the country is projected to grow from €72 million in 2019 to over €210 million by 2026, illustrating the positive impact of these regulatory advancements and the collaborative efforts of stakeholders, including bioaccess, in developing a cohesive strategy for the medical sector.
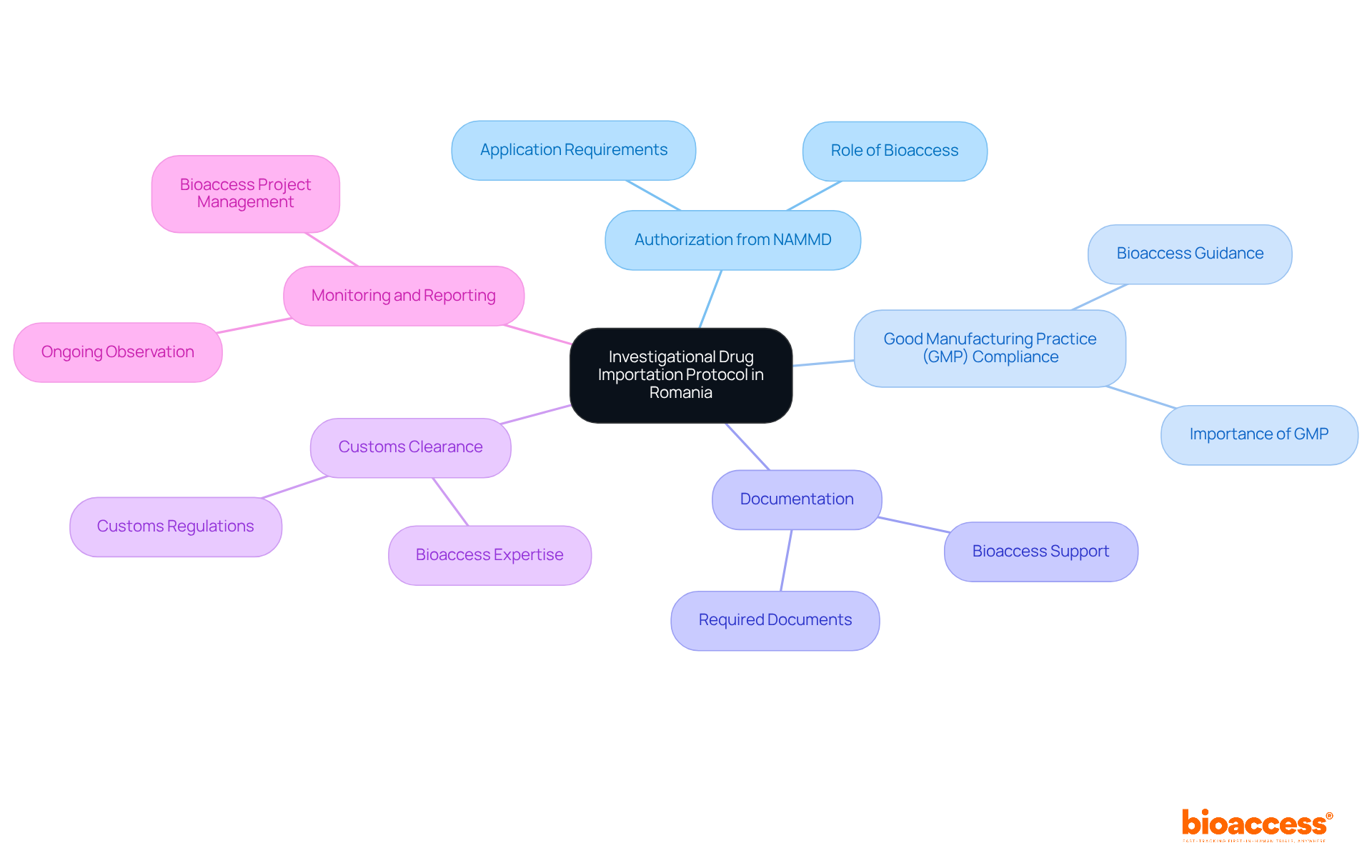
The Investigational Drug Importation Protocol in Romania encompasses several critical components and requirements:
Authorization from NAMMD: Sponsors must secure authorization from the National Agency for Medicines and Medical Devices (NAMMD) prior to importing any investigational medicinal product. This process requires a comprehensive application that details the product specifics, research protocol, and intended drug use. Bioaccess assists sponsors in preparing and submitting applications to comply with the investigational drug importation protocol in Romania and meet regulatory standards.
Good Manufacturing Practice (GMP) Compliance: All imported investigational drugs must adhere to GMP standards, which guarantee that products are manufactured in environments that meet stringent safety and quality criteria. In Romania, adherence to GMP is vital for upholding the integrity of research studies. Bioaccess provides guidance on the investigational drug importation protocol in Romania to ensure that all imported products comply with these critical GMP standards.
Documentation: Importers must provide several papers, including an import license, batch release certificate, and proof of ethical approval for the research study. Bioaccess assists clients in gathering and organizing documents necessary for compliance with the investigational drug importation protocol in Romania, ensuring that all regulatory requirements are met efficiently.
Customs Clearance: The importation process mandates adherence to customs regulations, which includes the submission of the Single Administrative Document (SAD) along with any other pertinent import documentation. Bioaccess facilitates this process by providing expertise in customs regulations, ensuring compliance with the investigational drug importation protocol in Romania for the legal entry of investigational drugs into the country.
Monitoring and Reporting: After the importation of the investigational drug, ongoing observation and documentation are crucial to guarantee adherence to the research protocol. This involves addressing any negative incidents that may happen during the study, thereby protecting participant safety and data integrity. Bioaccess offers comprehensive project management services to ensure compliance with the investigational drug importation protocol in Romania.
Romania's regulatory system promotes a clear and effective investigational drug importation protocol in Romania, enhancing the country's increasing reputation as an attractive site for research studies. With 2,618 studies presently registered, including 265 actively recruiting ones, the country provides a strong framework for medical investigations, marked by high patient availability and skilled staff.
The investigational drug importation protocol in Romania plays a crucial role in enhancing the effectiveness of research studies. By establishing a robust regulatory framework, the investigational drug importation protocol in Romania streamlines the importation process, allowing for quicker access to investigational drugs. This efficiency is particularly beneficial for pharmaceutical companies, as it significantly reduces the time and resources required to initiate studies.
Moreover, the protocol mandates that all imported medications adhere to strict safety and efficacy standards, ensuring patient welfare and maintaining the integrity of research. The country's alignment with EU regulations further boosts its appeal as a prime location for international studies, fostering collaboration among local and global stakeholders. Notably, the introduction of a 30-day silent approval mechanism has dramatically cut initiation times, enabling regulatory approvals in as little as 6-8 weeks, compared to the typical 6-12 months seen in other regions. This advancement not only accelerates the investigation process but also positions Romania as a competitive player in the global medical study landscape.
Additionally, bioaccess provides comprehensive clinical trial management services, including:
These services are vital for navigating the complexities of the regulatory environment and directly support the investigational drug importation protocol in Romania. This ultimately contributes to the growth of the medical study market in Romania, projected to expand from €72 million in 2019 to over €210 million by 2026.
In summary, the investigational drug importation protocol in Romania is pivotal in advancing medical research and improving patient outcomes. As the landscape evolves, collaboration among stakeholders will be essential to harness the full potential of these advancements.

The investigational drug importation protocol in Romania stands as a crucial regulatory framework, ensuring the safe and efficient entry of investigational medicinal products for clinical trials. By following this protocol, Romania not only bolsters its reputation as a significant player in the global research arena but also prioritizes patient safety and upholds ethical standards in medical studies.
Key aspects of the protocol include:
The streamlined processes and adherence to Good Manufacturing Practices (GMP) not only accelerate the importation of investigational drugs but also guarantee that they meet the highest safety and efficacy standards, ultimately benefiting both researchers and patients alike.
These insights reveal that the investigational drug importation protocol in Romania is far more than a regulatory formality; it is a cornerstone of effective clinical research. As the landscape of medical inquiry evolves, collaboration among stakeholders becomes essential to overcoming challenges and maximizing the potential for innovative therapies to reach patients swiftly. By emphasizing the significance of this protocol, we can foster ongoing advancements in clinical research, paving the way for Romania to solidify its status as a premier destination for pharmaceutical studies.
What is the investigational drug importation protocol in Romania?
The investigational drug importation protocol in Romania is a regulatory framework that governs the importation of investigational medicinal products (IMPs) for research trials, ensuring they meet safety and efficacy standards in accordance with national and European Union regulations.
Who oversees the investigational drug importation protocol in Romania?
The protocol is overseen by the National Agency for Medicines and Medical Devices (NAMMD), which authorizes the importation process and ensures compliance with Good Clinical Practice (GCP) guidelines.
What services does bioaccess offer related to the investigational drug importation protocol in Romania?
Bioaccess provides various research study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting.
What are the advantages of conducting medical studies in Romania?
Romania offers significant advantages such as high patient availability and expedited recruitment timelines, which are critical for the success of medical investigations.
How many studies are currently registered in Romania, and how many are actively recruiting?
There are currently 2,618 studies registered in Romania, with 265 actively recruiting investigations.
What is the timeline for research approvals under the investigational drug importation protocol in Romania?
The regulatory body provides feedback on submissions within 10 days and authorization within a maximum of 60 days, facilitating the prompt commencement of studies.
Why is the investigational drug importation protocol important for clinical research?
It regulates the entry of new therapeutic products into Romania, ensuring secure importation and accelerating research studies that assess the safety and effectiveness of new treatments, thereby enhancing the country's reputation as a hub for medical studies.
How does adherence to the protocol impact patient safety and public confidence?
Compliance with the investigational drug importation protocol is essential for maintaining the integrity of medical studies and safeguarding patient safety, which in turn boosts public confidence in the investigative process.
What impact does the protocol have on the speed of commencing medical studies?
The transparency and efficiency of the investigational drug importation protocol significantly impact how quickly medical studies can commence, making Romania an attractive destination for pharmaceutical firms.
How does the investigational drug importation protocol contribute to innovative therapies reaching patients?
It remains a cornerstone for ensuring that innovative therapies are imported safely and efficiently, enhancing the research landscape and fostering collaboration among stakeholders in clinical research.