


The National Medical Products Administration (NMPA) is pivotal in regulating pharmaceuticals, healthcare devices, and cosmetics in China, ensuring their safety, efficacy, and quality throughout their lifecycle. This oversight not only safeguards public health but also fosters innovation, positioning China as a competitive player in the global pharmaceutical market. The NMPA's expedited approval pathways and stringent compliance standards illustrate its comprehensive approach to regulation.
By addressing these key areas, the NMPA enhances the credibility of medical products, thereby encouraging stakeholders to engage actively in the clinical research landscape. The importance of collaboration in this context cannot be overstated, as it is essential for advancing medical technologies and addressing the challenges within the industry.
The National Medical Products Administration (NMPA) serves as a critical player in China's healthcare landscape, responsible for ensuring the safety and efficacy of medical products, ranging from pharmaceuticals to medical devices. As the regulatory framework evolves, the NMPA's initiatives not only bolster public health but also stimulate innovation within the industry, resulting in an increase in new product approvals. However, the complexities of compliance and the demanding approval processes present significant challenges for manufacturers. How does the NMPA reconcile stringent regulations with the pressing need for timely access to innovative healthcare solutions?
The national medical product administration serves as China's principal regulatory authority, overseeing the safety, efficacy, and quality of pharmaceuticals, healthcare devices, and cosmetics. Established under the national medical product administration, the agency's mission encompasses the entire lifecycle of health products—from development and registration to post-market surveillance. This comprehensive oversight by the national medical product administration is essential for safeguarding public health and ensuring that only safe and effective healthcare products reach consumers.
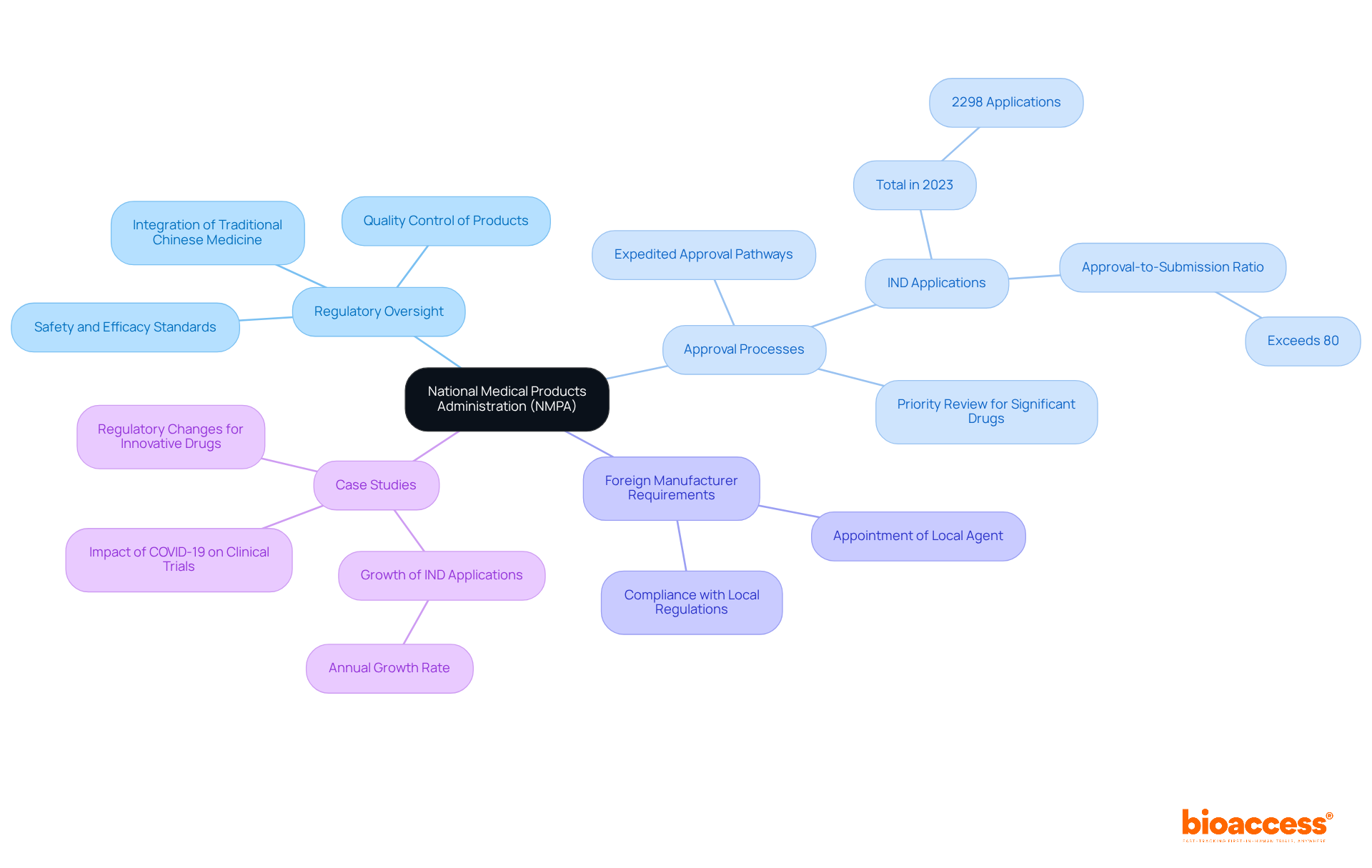
The national medical product administration has significantly shaped the regulatory framework of the medical product landscape in China, enhancing safety standards and fostering innovation. Notably, the national medical product administration has introduced expedited approval pathways for innovative drugs, resulting in a remarkable surge in Investigational New Drug (IND) applications—2298 in 2023 alone, with an impressive approval-to-submission ratio exceeding 80%. This illustrates the national medical product administration's commitment to maintaining a balance between rigorous oversight and the urgent need for timely access to new treatments.
Foreign manufacturers aiming to register products with the national medical product administration in China are required to appoint a local agent, a crucial step for navigating the complex regulatory environment. Firms like bioaccess® facilitate this process by connecting Medtech, Biopharma, and Radiopharma startups with leading clinical research sites, enabling them to initiate trials 40% faster while ensuring compliance with relevant regulations. This integration of services is essential for meeting the requirements of the national medical product administration and expediting the approval process.
Case studies underscore the effectiveness of the national medical product administration in regulating complex products, such as biologics and oncology drugs, which have garnered special focus due to their significant therapeutic value. The national medical product administration's evolving regulatory practices not only bolster patient safety but also position China as a formidable competitor in the global pharmaceutical arena.
Established in 1998 as the State Drug Administration (SDA), the National Medical Products Administration primarily focuses on drug regulation. In 2013, it rebranded as the national medical product administration (formerly known as the China Food and Drug Administration), reflecting a broader scope of responsibilities. A crucial change occurred in 2018 when the CFDA was rebranded as the national medical product administration, reflecting its expanded authority to supervise not only pharmaceuticals but also devices and cosmetics. This evolution was driven by the necessity to enhance oversight in line with the national medical product administration, improve public health outcomes, and align with international standards.
Since its inception, the national medical product administration has enacted considerable reforms, particularly in 2025, aimed at simplifying oversight processes and promoting innovation within the device industry. Notably, the annual number of new drug approvals surged to a record high of 70 in 2021, showcasing the impact of these reforms by the national medical product administration. Additionally, the median new drug application approval time decreased to 15.4 months from 2017 to 2021, which reflects the national medical product administration's commitment to efficiency and responsiveness in drug regulation.
The agency's journey from the SDA to its current position exemplifies a proactive strategy for adapting to the dynamic environment of healthcare and the oversight requirements set by the national medical product administration in China.

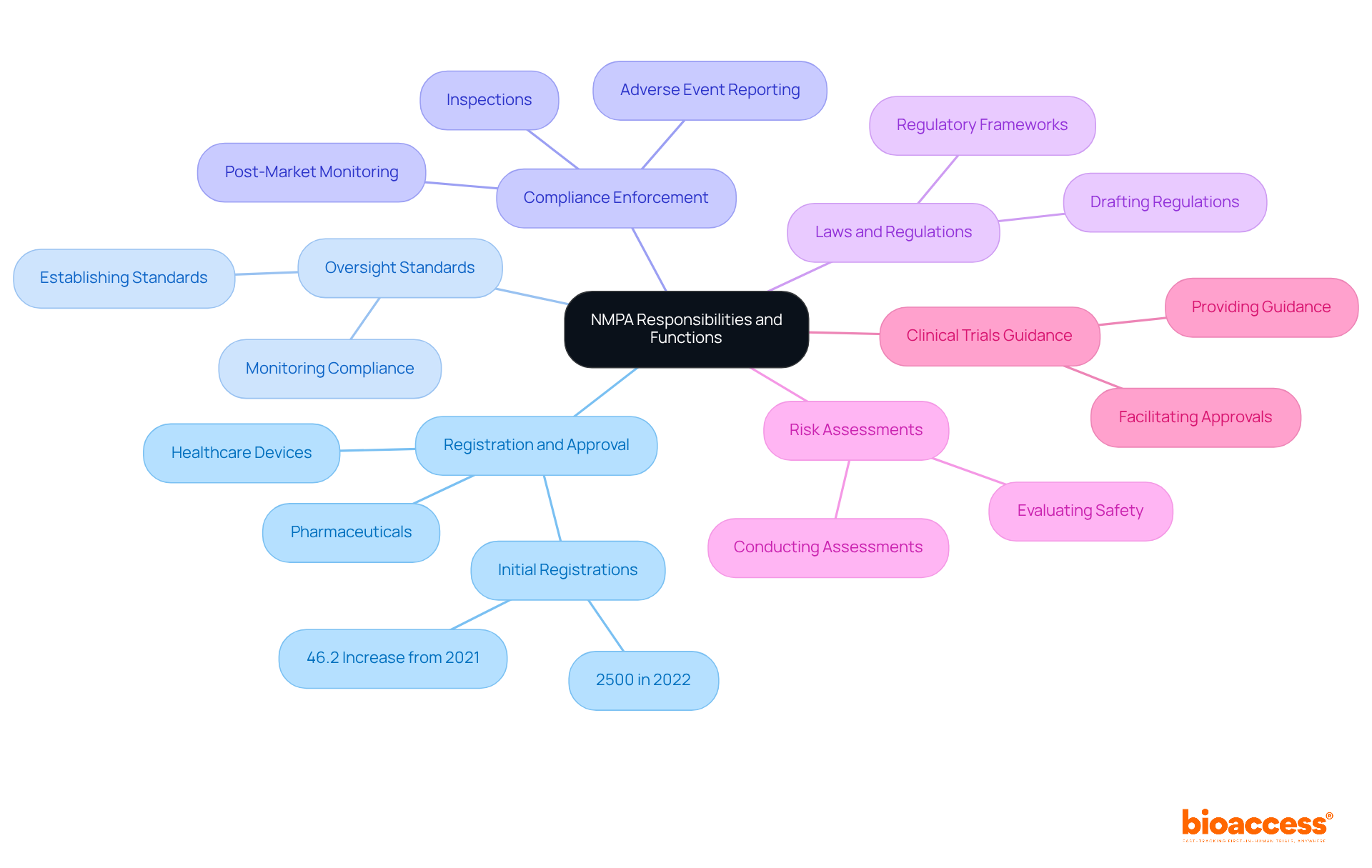
The national medical product administration plays a vital role in overseeing health-related products in China, encompassing a broad range of responsibilities. These include:
Joint initiatives with manufacturers, healthcare providers, and global regulatory agencies ensure that health products meet stringent safety and efficacy standards.
In recent years, the national medical product administration has demonstrated its commitment to enhancing the healthcare device sector by facilitating faster approvals for innovative technologies. Notably, the approval rate for new medical devices has experienced significant growth, with initial registrations reaching 2,500 in 2022, reflecting a 46.2% increase from the previous year. This surge underscores the agency's focus on promoting innovation while maintaining high compliance standards.
Furthermore, the national medical product administration (NMPA) demonstrates its enforcement of compliance through post-marketing obligations, which require manufacturers to monitor adverse events and ensure the continued safety of their products. The agency's proactive approach in conducting inspections and evaluations further solidifies its role in safeguarding public health. Expert opinions highlight that the agency's regulatory framework is evolving, aligning with global standards to enhance the quality and safety of healthcare products in the market. This evolution is crucial as China positions itself as a competitive player in the global pharmaceutical landscape.
The guidelines established by the national medical product administration play a crucial role in shaping the healthcare device sector in China. By enforcing stringent standards for safety and efficacy, the regulatory authority ensures that only high-quality products are available to consumers, significantly enhancing patient safety. However, these regulations also pose considerable challenges for manufacturers, including protracted approval timelines and complex compliance requirements that vary based on device classification. For instance, the typical approval duration for health-related devices under regulatory guidelines can exceed six months, particularly for Class III products, which necessitate more comprehensive documentation and testing.
Recent initiatives by the national medical product administration are focused on streamlining the approval process and fostering innovation, which has resulted in improved market access for both foreign and domestic companies. The emphasis of the national medical product administration on administrative transparency and collaboration has led to a notable increase in the registration of innovative items, with over 126,000 medical device entries recorded by the end of 2024. This surge reflects a growing trend where manufacturers are adapting to regulatory demands by enhancing their offerings to align with both local and international standards.
Expert opinions indicate that while the national medical product administration's rigorous framework can be intimidating, it ultimately compels manufacturers to innovate and enhance their products. For example, recent modifications to the registration application file requirements have reduced barriers for domestic registration, promoting the localization of advanced healthcare equipment. Consequently, the medical device industry in China is not only expanding rapidly but is also evolving into a hub for innovative solutions that satisfy the changing needs of healthcare providers and patients alike.
In Colombia, navigating similar governance environments is essential for success. Experts like Ana Criado, Director of Regulatory Affairs at bioaccess, underscore the significance of comprehensive clinical trial management services, which encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. Drawing from her extensive background in regulatory affairs and biomedical engineering, Ana offers valuable insights into how companies can effectively manage these challenges and align their strategies with both local and international regulations.
The National Medical Products Administration (NMPA) stands as a cornerstone in safeguarding the safety and efficacy of healthcare products in China. By overseeing the complete lifecycle of pharmaceuticals, medical devices, and cosmetics, the NMPA plays a pivotal role in protecting public health while simultaneously fostering innovation within the industry.
Throughout its evolution, transitioning from the State Drug Administration to its current form, the NMPA has enacted significant reforms that streamline regulatory processes and elevate safety standards. This agency's unwavering commitment to efficiency is evident in the remarkable surge of drug approvals and the increasing influx of innovative medical devices entering the market. These advancements not only reflect the NMPA's dedication to maintaining high compliance standards but also position China as a formidable player in the global pharmaceutical landscape.
As the NMPA continues to adapt to the dynamic healthcare environment, its influence on the medical device industry cannot be overstated. Manufacturers are encouraged to innovate and align their products with stringent safety regulations, ultimately benefiting both patients and healthcare providers. Embracing this regulatory framework is essential for companies aiming to thrive in the rapidly evolving market, underscoring the critical importance of understanding and navigating the NMPA's guidelines for future success.
What is the National Medical Products Administration (NMPA)?
The NMPA is China's principal regulatory authority responsible for overseeing the safety, efficacy, and quality of pharmaceuticals, healthcare devices, and cosmetics.
What is the mission of the NMPA?
The mission of the NMPA encompasses the entire lifecycle of health products, including development, registration, and post-market surveillance, to safeguard public health and ensure that only safe and effective products reach consumers.
How has the NMPA impacted the regulatory framework in China?
The NMPA has enhanced safety standards, fostered innovation, and introduced expedited approval pathways for innovative drugs, significantly shaping the medical product landscape in China.
What was the number of Investigational New Drug (IND) applications in 2023, and what is the approval-to-submission ratio?
In 2023, there were 2,298 IND applications, with an approval-to-submission ratio exceeding 80%.
What is required of foreign manufacturers to register products with the NMPA?
Foreign manufacturers must appoint a local agent to navigate the complex regulatory environment in China.
How do firms like bioaccess® assist in the registration process with the NMPA?
Firms like bioaccess® connect Medtech, Biopharma, and Radiopharma startups with leading clinical research sites, enabling them to initiate trials 40% faster while ensuring compliance with relevant regulations.
What types of products have received special focus from the NMPA?
The NMPA has placed special focus on regulating complex products, such as biologics and oncology drugs, due to their significant therapeutic value.
How do the NMPA's regulatory practices affect patient safety and the global pharmaceutical market?
The NMPA's evolving regulatory practices bolster patient safety and position China as a formidable competitor in the global pharmaceutical arena.