


The role of a clinical monitor in trials is crucial for ensuring compliance with study protocols, safeguarding participant safety, and maintaining the integrity of research data. Clinical monitors, also known as Clinical Research Associates (CRAs), are pivotal in overseeing trials through rigorous monitoring, ongoing training, and effective communication. This comprehensive approach not only enhances data quality but also significantly reduces the risks associated with medical research. By emphasizing the importance of these roles, we can better appreciate the critical contributions of clinical monitors to the success of clinical trials.
Understanding the complexities of clinical trials reveals a pivotal figure often working behind the scenes: the clinical monitor. These professionals are essential in ensuring that research studies adhere to ethical standards and regulatory requirements, ultimately safeguarding participant safety and data integrity. However, as trials become more intricate and compliance challenges persist, what does it truly take for clinical monitors to excel in their roles? This article delves into the multifaceted responsibilities and evolving practices of clinical monitors, highlighting their indispensable impact on the success of clinical research.
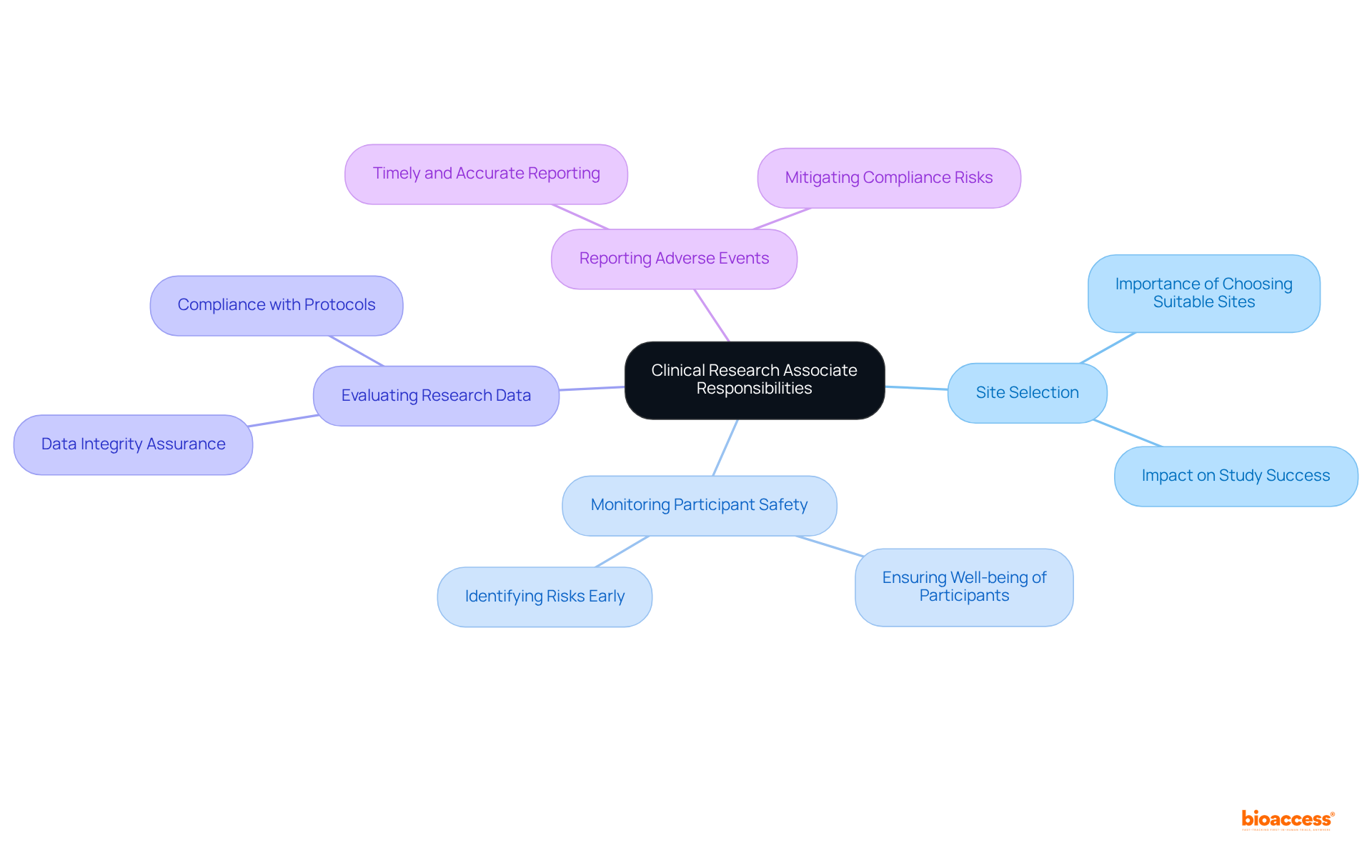
A Clinical Research Associate (CRA) plays a crucial role in overseeing research studies, ensuring compliance with study protocols, regulatory standards, and Good Clinical Practice (GCP) guidelines. Acting as the primary liaison between the sponsor and the investigator, CRAs provide essential support to guarantee that studies are conducted and documented accurately. Their key responsibilities encompass:
Statistics reveal that compliance issues remain a significant barrier in research studies, with findings indicating that the average evaluation scores for CRAs were only 60%. This statistic underscores the urgent need for ongoing training and a steadfast commitment to best practices. By maintaining vigilant oversight throughout the study process, CRAs are instrumental in safeguarding both the integrity of the collected data and the well-being of participants. Their involvement is vital in identifying and mitigating compliance risks, ultimately contributing to the success of medical studies.
Oversight professionals are essential to the success of research trials, as they ensure ethical practices and compliance with regulatory standards. Their supervision significantly mitigates risks associated with medical research, particularly concerning information integrity and participant safety.
Through regular site visits and evaluations, the clinical monitor can swiftly identify potential issues, allowing for prompt actions that enhance data quality and participant well-being. This proactive approach not only bolsters the reliability of the collected data but also fosters trust among stakeholders, including sponsors, regulatory bodies, and participants.
Ongoing observation has been shown to substantially reduce the incidence of severe adverse events, thereby improving the overall safety record of research studies. By maintaining rigorous oversight, healthcare reviewers play a pivotal role in advancing medical knowledge and enhancing patient outcomes, underscoring their indispensable function in the research landscape.
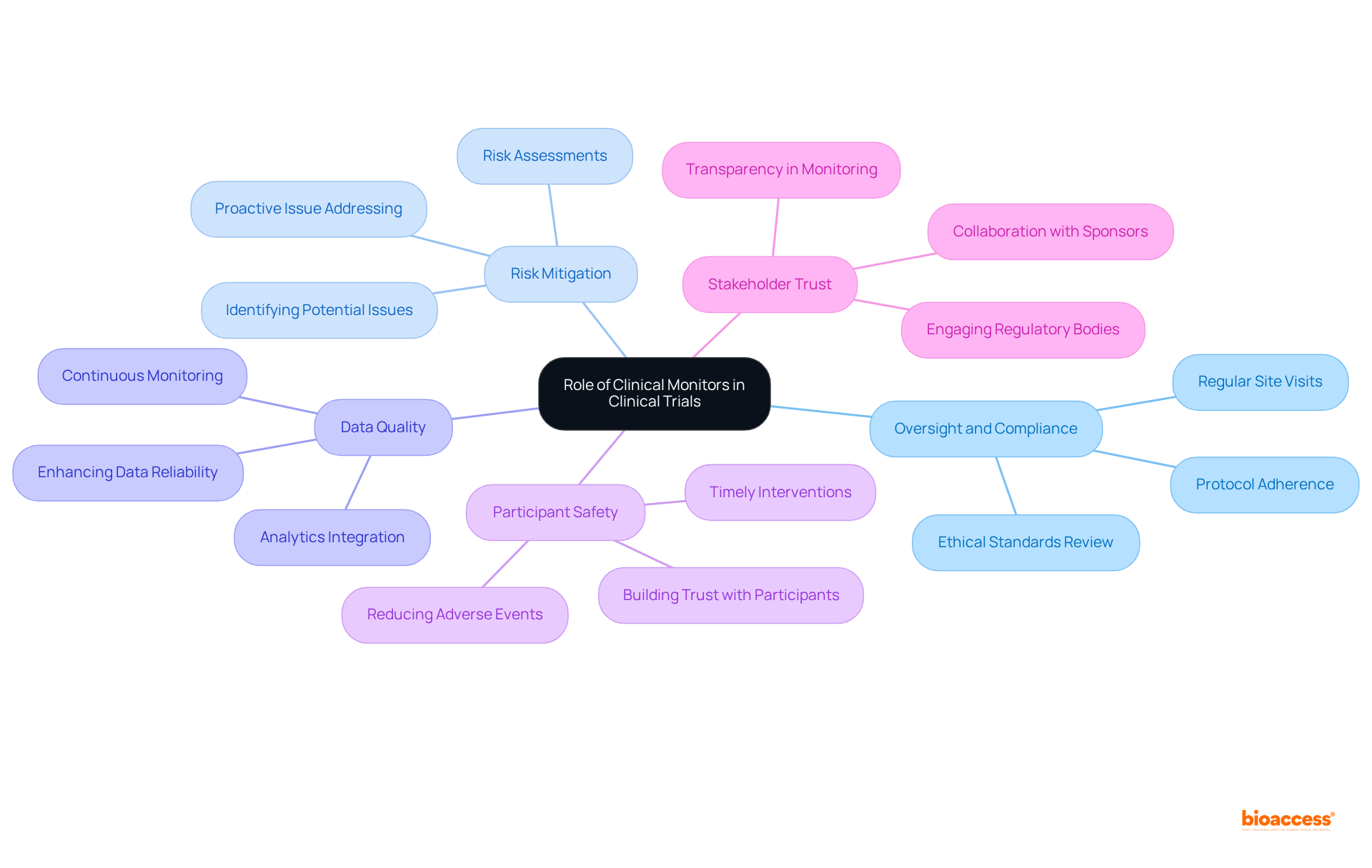
The role of medical monitors has undergone a profound transformation since the early phases of research studies, which often suffered from inadequate oversight and significant ethical dilemmas.
The introduction of Good Clinical Practice (GCP) guidelines in the late 20th century marked a crucial turning point, underscoring the need for rigorous monitoring to safeguard participants and uphold the integrity of data.
As medical studies have evolved, so too have the methodologies employed in monitoring.
The emergence of sophisticated technologies has revolutionized these practices, enabling remote monitoring and the implementation of risk-based strategies that markedly improve both efficiency and effectiveness.
Today, research supervisors utilize advanced tools for real-time data analysis, fostering enhanced communication with trial sites.
This continuous evolution underscores the vital importance of clinical monitoring in ensuring the reliability and safety of clinical research.
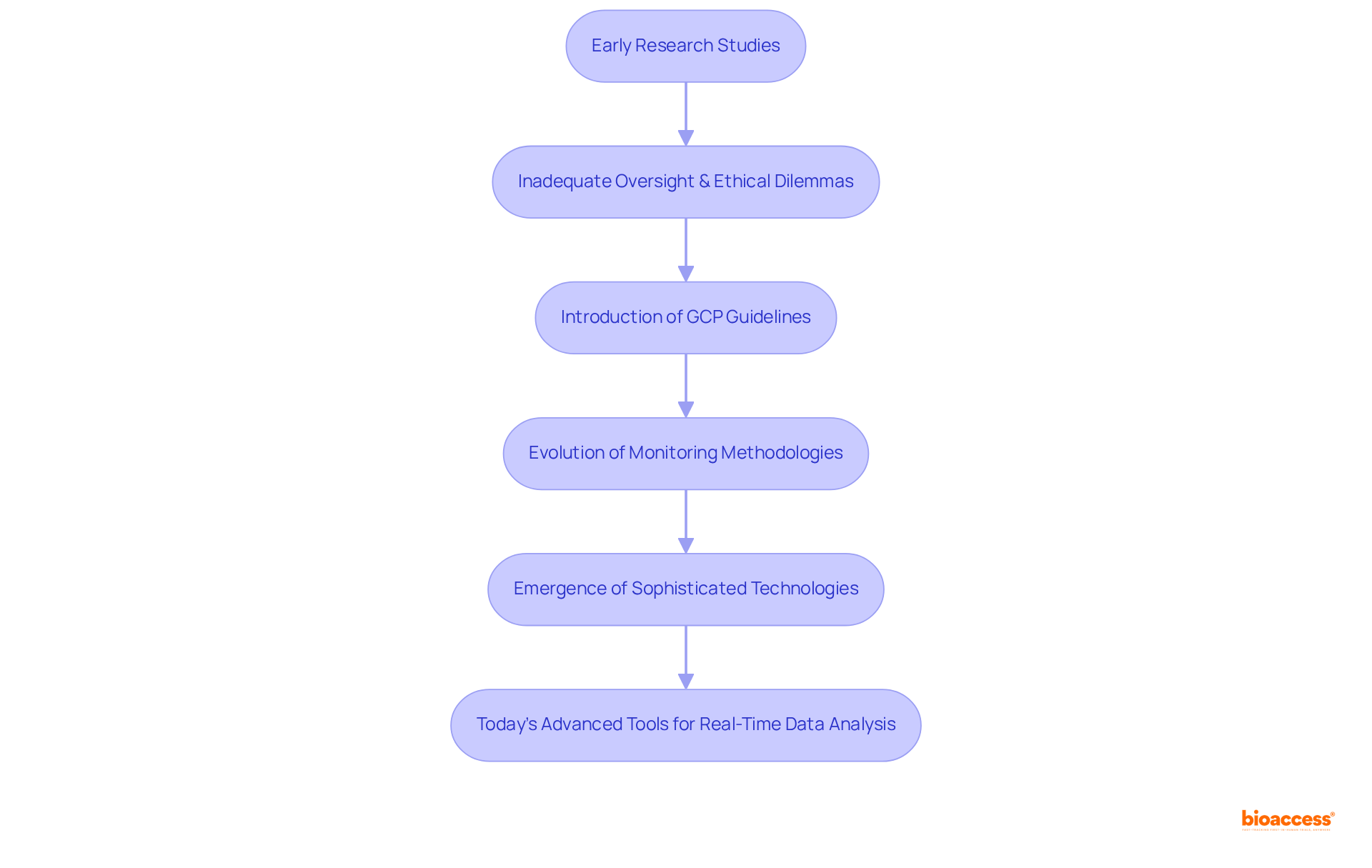
To excel as a research supervisor, individuals typically require a background in life sciences, nursing, or a related field, often holding at least a bachelor's degree. Key skills encompass:
A profound understanding of regulatory requirements and Good Clinical Practice (GCP) guidelines is essential for a clinical monitor. Training programs and certifications, such as those offered by the Association of Clinical Research Professionals (ACRP) and the Society of Clinical Research Associates (SOCRA), play a pivotal role in equipping research supervisors with the necessary skills. Ongoing professional development is crucial, as it enables evaluators to stay abreast of evolving regulations and optimal research methodologies.
Notably, organizations that promote collaborative working are five times more likely to achieve high performance, underscoring the significance of teamwork in this field. Furthermore, statistics reveal that 80% of research studies face delays due to recruitment challenges, highlighting the necessity for skilled supervisors who can adeptly navigate these complexities.
bioaccess® accelerates studies by providing ethical approvals in 4-6 weeks and achieving enrollment 50% faster than conventional markets, showcasing the effectiveness that a clinical monitor brings to the research process. As Christine Pfund emphasized, effective mentoring is crucial for the success of early-career researchers, underscoring the importance of training and guidance in cultivating skilled professionals.
With bioaccess®'s comprehensive services, including pre-qualified networks for swift site activation, research supervisors can leverage streamlined processes to enhance study outcomes and assist Medtech, Biopharma, and Radiopharma startups in navigating the intricacies of research.
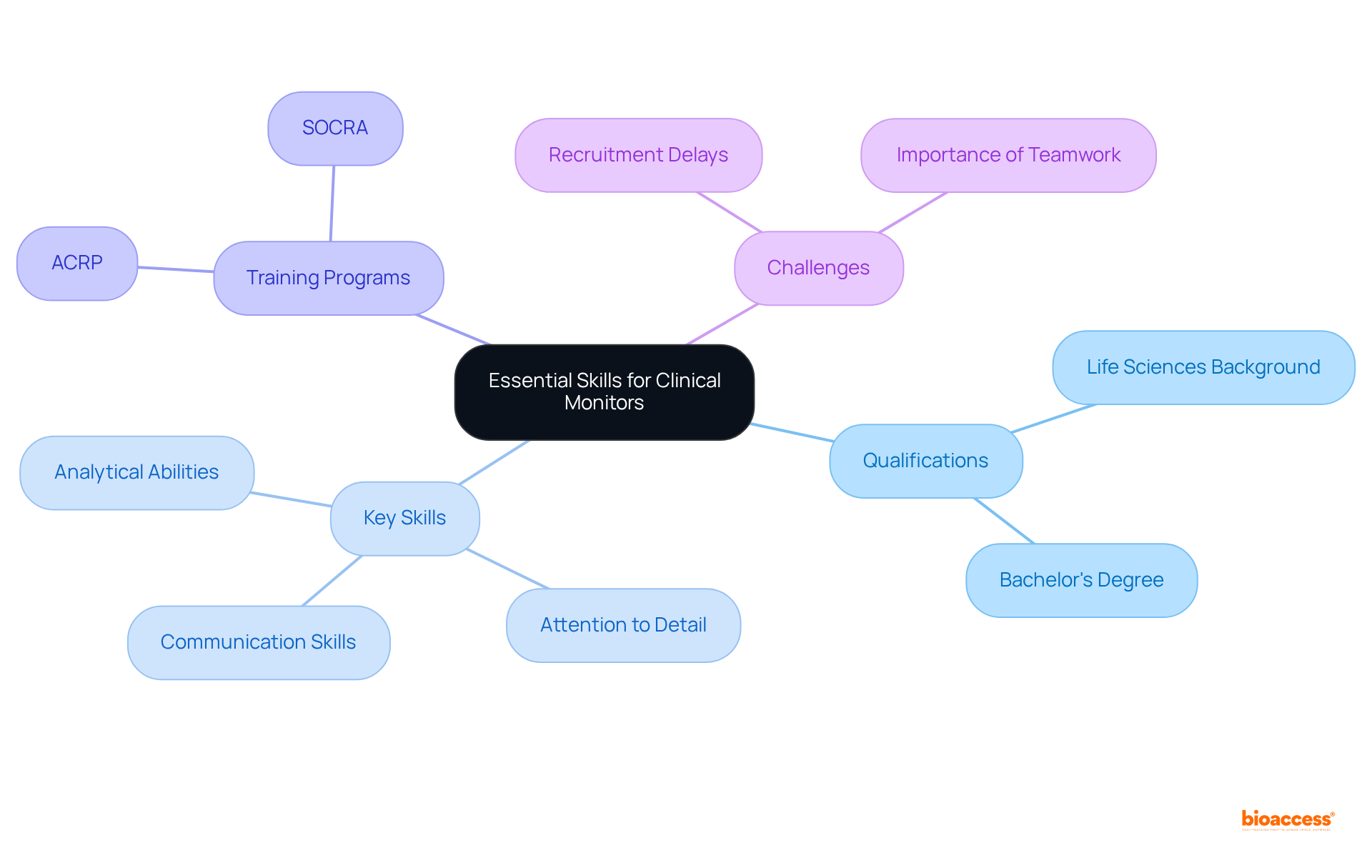
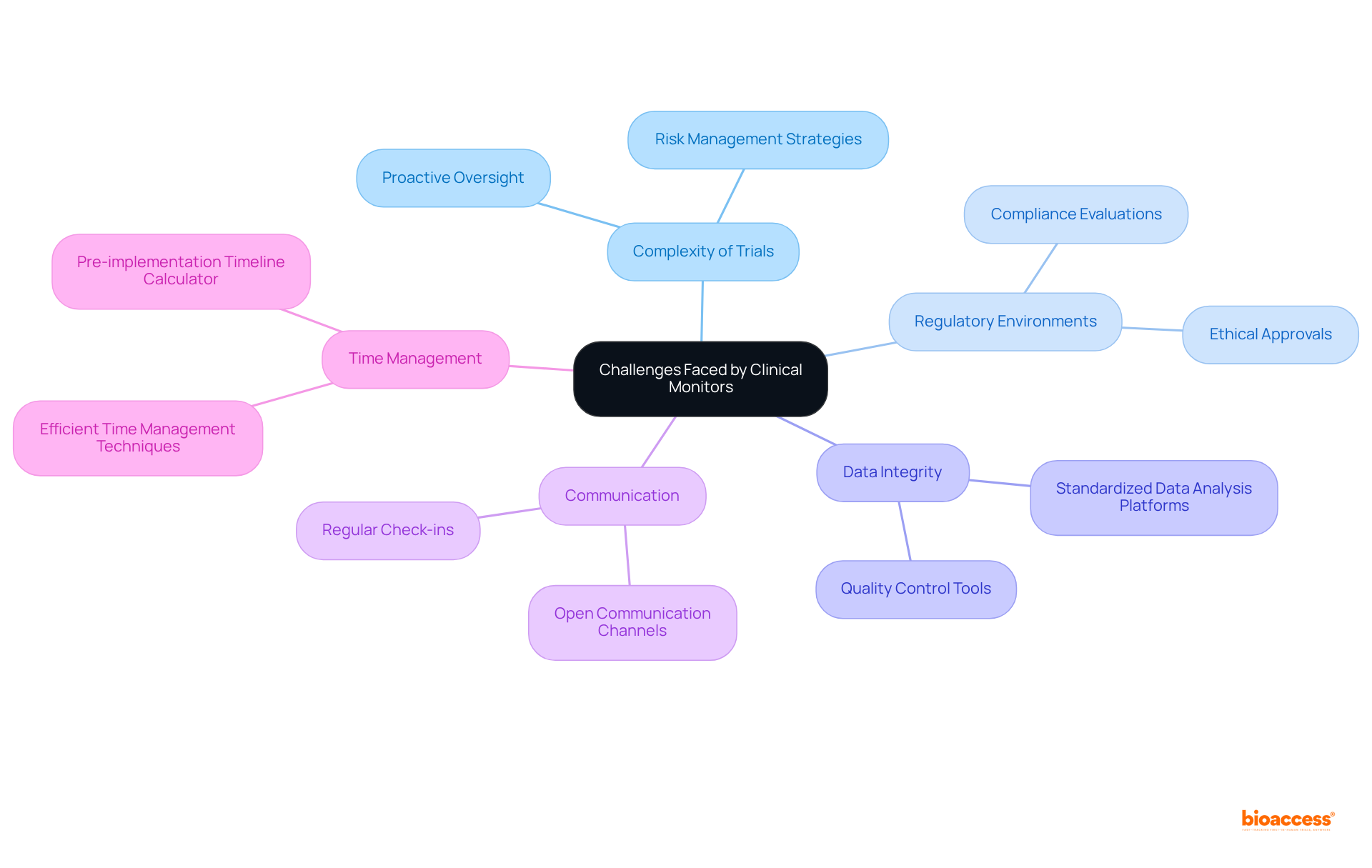
Clinical monitors face a variety of challenges in their roles, especially when overseeing multiple clinical studies concurrently. The complexity of these trials, coupled with varying regulatory environments, necessitates a strong focus on adherence and participant safety. As studies become increasingly intricate and technology evolves rapidly, the clinical monitor plays a crucial role in maintaining data integrity and facilitating effective communication with research sites, which can prove to be daunting tasks. Monitors must remain vigilant against potential protocol deviations and adverse events, which demands proactive oversight and a comprehensive risk management strategy.
To navigate these challenges effectively, a clinical monitor employs robust organizational skills and efficient time management. They often utilize standardized data analysis platforms to reduce errors and enhance collaboration among study teams. For instance, the implementation of tools like the Pre-implementation Timeline Calculator has shown promise in streamlining the pre-implementation phase of multi-site studies, significantly reducing the time and financial resources needed for setup.
Furthermore, fostering open communication channels with site teams is essential. Engaging in regular check-ins and leveraging learning networks can aid in addressing compliance issues and sharing best practices, ultimately leading to improved study management. By prioritizing participant safety and adhering to ethical guidelines, research overseers play a pivotal role in upholding the integrity of studies and ensuring the successful advancement of medical products.
bioaccess® offers comprehensive management services for studies, including:
These services are designed to directly alleviate the challenges faced by clinical monitors by providing structured support and resources. As Diana Davydov, MD, emphasizes, "Medical Monitors are a critical component of the clinical trial process, providing valuable insights into the safety and efficacy of medical products under development.
The pivotal role of clinical monitors in clinical trials is undeniably significant. Serving as the linchpin between sponsors and investigators, these professionals ensure that research studies comply with rigorous protocols and regulatory standards. Their unwavering commitment to participant safety and data integrity is essential for the successful execution of clinical trials, rendering them indispensable in the research process.
This article explored the key responsibilities of clinical monitors, emphasizing their involvement in:
The evolution of their role, shaped by the introduction of Good Clinical Practice guidelines and advancements in technology, highlights the increasing complexity and importance of effective monitoring. Furthermore, the essential skills and ongoing training required for clinical monitors underscore the necessity of a well-prepared workforce capable of navigating the challenges inherent in clinical research.
Reflecting on the significance of clinical monitors, it is evident that their rigorous oversight not only enhances the reliability of research outcomes but also fosters trust among all stakeholders involved. As the landscape of clinical trials continues to evolve, prioritizing the development and support of skilled clinical monitors is crucial. By investing in their training and resources, the medical research community can ensure the ethical advancement of medical knowledge and improved patient outcomes.
What is the role of a Clinical Research Associate (CRA)?
A CRA oversees research studies, ensuring compliance with study protocols, regulatory standards, and Good Clinical Practice (GCP) guidelines. They act as the primary liaison between the sponsor and the investigator.
What are the key responsibilities of a Clinical Monitor?
Key responsibilities include site selection, monitoring participant safety, evaluating research data, and ensuring timely and accurate reporting of adverse events.
Why is compliance important in clinical research?
Compliance is crucial because it safeguards the integrity of the collected data and the well-being of participants. Non-compliance can lead to significant barriers in research studies.
What statistic highlights the need for ongoing training for CRAs?
Statistics indicate that the average evaluation scores for CRAs were only 60%, underscoring the need for ongoing training and a commitment to best practices.
How do clinical monitors contribute to participant safety?
Clinical monitors conduct regular site visits and evaluations to identify potential issues quickly, which allows for prompt actions that enhance participant safety and data quality.
What impact does ongoing observation by clinical monitors have on research studies?
Ongoing observation has been shown to substantially reduce the incidence of severe adverse events, improving the overall safety record of research studies.
How do clinical monitors foster trust among stakeholders?
By maintaining rigorous oversight and ensuring ethical practices, clinical monitors foster trust among sponsors, regulatory bodies, and participants, thereby enhancing the reliability of the collected data.