


The role of a Principal Investigator (PI) in clinical trials is paramount, as they oversee the study's protocol and ensure ethical standards, regulatory compliance, and participant safety throughout the research process. PIs are tasked with several key responsibilities, including:
These functions collectively uphold the integrity and success of clinical trials, highlighting the critical nature of their position. Understanding the multifaceted role of PIs is essential for appreciating the complexities of clinical research and the necessity for skilled leadership.
The Principal Investigator (PI) serves as the linchpin in clinical trials, wielding authority and responsibility that significantly shape the outcomes of medical research. In an evolving landscape of clinical studies, particularly with the integration of advanced technologies, the role of the PI has become increasingly complex and vital. What challenges do PIs face in this dynamic environment? How do their skills and leadership directly influence the integrity and success of clinical trials? Exploring these questions reveals not only the multifaceted duties of PIs but also underscores the critical importance of their role in advancing healthcare innovation.
The Principal Investigator (PI) clinical trial plays a pivotal role in executing research studies, serving as the primary authority at a specific site. This position entails comprehensive oversight of the study protocol, encompassing preparation, execution, and management, all while ensuring adherence to regulatory standards and ethical guidelines. Typically a certified medical expert or investigator, the PI clinical trial leader supervises the study team, overseeing all facets including participant recruitment, data collection, analysis, and reporting. Their leadership is essential for maintaining study integrity and safeguarding participant welfare throughout the PI clinical trial investigation.
As we approach 2025, the responsibilities of PIs have notably expanded, reflecting the industry's transition towards digital transformation. PIs are now expected to seamlessly integrate advanced technologies, such as remote monitoring tools and electronic health records, into their workflows. This evolution not only enhances data precision but also streamlines participant engagement, facilitating real-time monitoring of study progress.
Successful case studies illustrate the significant impact of effective PIs in clinical trials on research outcomes. For instance, PIs who adeptly manage multidisciplinary teams and maintain clear communication with sponsors and regulatory bodies substantially improve the efficiency and compliance of the PI clinical trial. Their ability to navigate complex regulatory landscapes while prioritizing participant safety is vital for the successful execution of PI clinical trials.
Expert opinions highlight the critical role of PIs in the PI clinical trial, which ensures ethical conduct and the generation of high-quality data. They are tasked with securing Institutional Review Board (IRB) approvals, monitoring adverse events, and ensuring informed consent, all of which are essential for preserving participant trust and regulatory compliance. As the cornerstone of medical studies, PIs are instrumental in advancing medical knowledge and developing innovative treatments through the PI clinical trial.
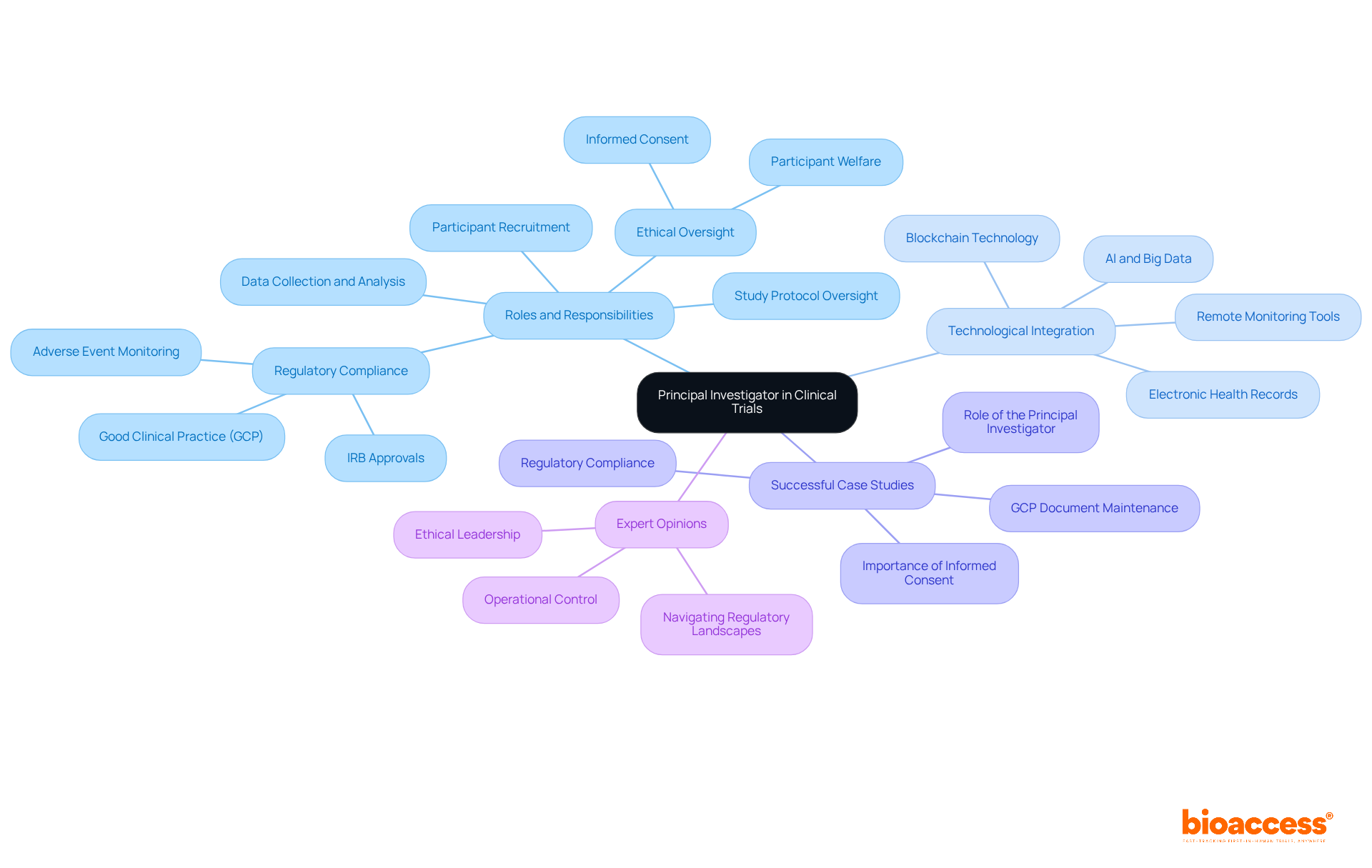
The duties of a Principal Investigator (PI) are broad and crucial to the success of the PI clinical trial. Key duties include:
Study Design and Protocol Development: The PI is tasked with designing the study and developing the protocol, ensuring it is both scientifically robust and ethically sound. This foundational step is crucial for the integrity of the research.
Regulatory Compliance: Ensuring compliance with all applicable regulations and guidelines is a primary responsibility. This involves acquiring required permissions from Institutional Review Boards (IRBs) and following Good Clinical Practice (GCP) standards, which are crucial for upholding ethical integrity during the study. Bioaccess supports PIs by providing comprehensive review and feedback on study documents to ensure compliance with country requirements.
Participant Recruitment and Informed Consent: The PI oversees participant recruitment, ensuring that informed consent is obtained effectively. This procedure necessitates transparent communication regarding the study's risks and benefits, as insufficient informed consent can result in significant ethical breaches and possible suspension of the study. Recruitment and retention of participants is a persistent hurdle for PIs, impacting the overall success of the research. Bioaccess tackles these challenges by applying innovative strategies to improve patient recruitment, especially for PI clinical trials.
Data Management and Integrity: Preserving the integrity of the data gathered during the study is essential. The PI is accountable for guaranteeing precise reporting and strict compliance with the study protocol, which is essential for the credibility of study results. PIs must maintain audit-ready records throughout the study to ensure compliance during audits.
Team Leadership: Guiding and overseeing the group is a significant aspect of the PI's role. This includes delegating tasks appropriately and fostering effective communication among team members to ensure smooth operations and collaboration. Professional, timely communication with sponsors and CROs is not just a courtesy — it is a regulatory expectation.
Monitoring and Reporting: The PI continuously observes the study's progress, addressing any emerging issues promptly. They are also accountable for communicating results to stakeholders and regulatory entities, ensuring transparency and adherence throughout the study process. Timely reporting of Serious Adverse Events (SAEs) is essential to uphold participant safety and regulatory standards. Bioaccess also provides project management and monitoring services to assist PIs in these responsibilities.
In 2025, the role of the PI clinical trial is more crucial than ever, as they navigate intricate regulatory environments and utilize technological advancements to enhance management and participant safety. As one Principal Investigator noted, "Every decision — from stopping rules to unblinding triggers — requires swift, evidence-based judgment." This emphasizes the significance of the PI's role in guaranteeing ethical, high-quality medical studies, particularly in partnership with organizations like Bioaccess, which seek to establish areas such as Barranquilla as premier locations for medical experimentation in Latin America.

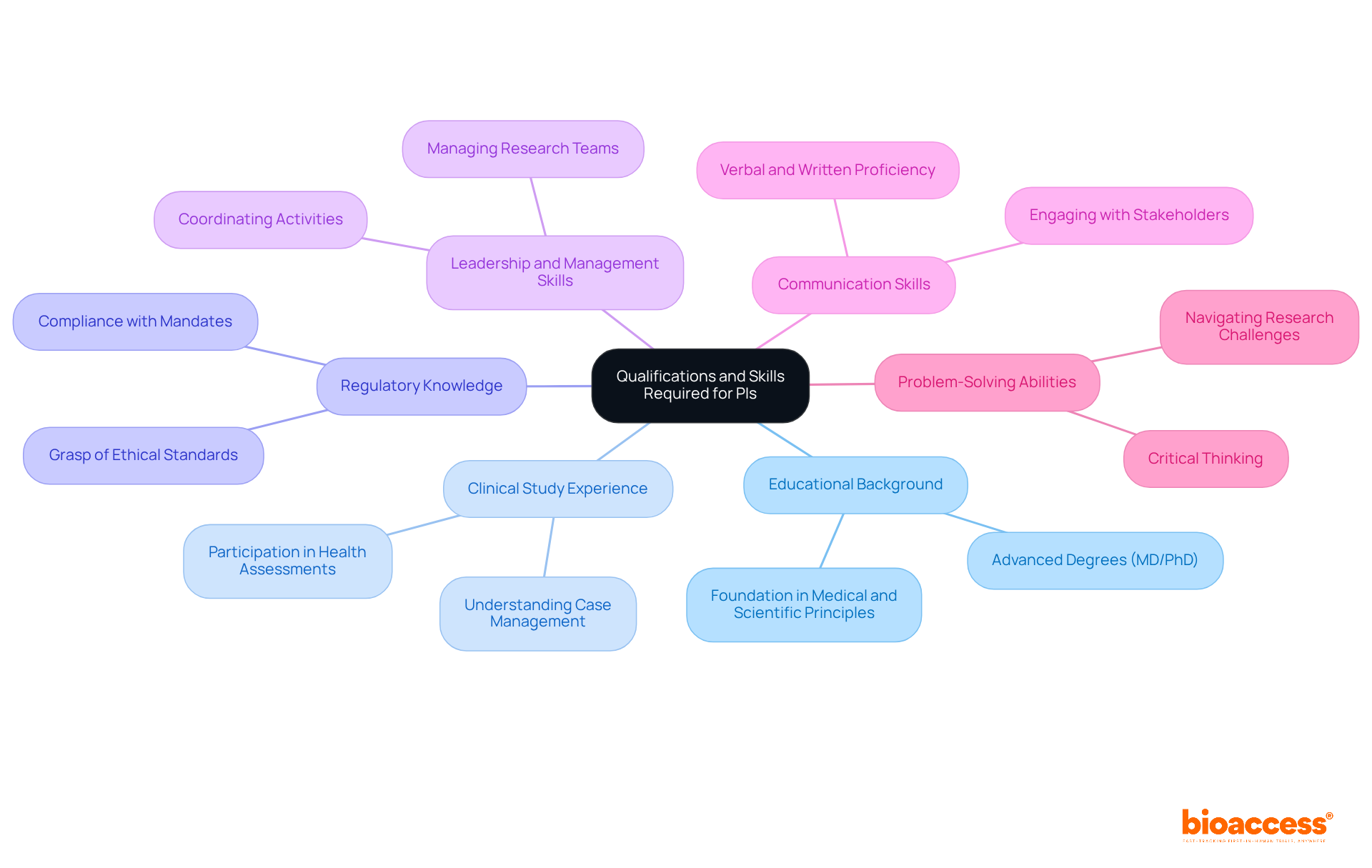
To become a Principal Investigator (PI), individuals typically need a blend of education, experience, and specific skills:
Educational Background: Most PIs hold advanced degrees, such as a Doctor of Medicine (MD) or Doctor of Philosophy (PhD), which provide a solid foundation in medical and scientific principles.
Clinical Study Experience: A strong foundation in medical studies is vital, frequently requiring previous participation in performing health assessments or associated study activities. This experience is essential for comprehending the intricacies of case management.
Regulatory Knowledge: PIs must have a thorough grasp of the regulatory environment, including ethical standards and compliance mandates that oversee the PI clinical trial. This knowledge is essential for ensuring that assessments comply with established standards.
Leadership and Management Skills: Effective leadership is paramount, as PIs are responsible for managing research teams, coordinating activities, and fostering collaboration among team members. Effective leadership can greatly influence success in experiments and team dynamics.
Communication Skills: Proficient verbal and written communication skills are necessary for engaging with participants, stakeholders, and regulatory bodies. Clear communication helps in conveying complex information and ensuring all parties are aligned.
Problem-Solving Abilities: PIs should exhibit critical thinking and problem-solving skills to effectively navigate challenges that may occur during the research process. The capability to resolve problems quickly is essential for upholding study integrity and participant safety.
In 2025, the need for PIs with these abilities in the context of PI clinical trials is anticipated to increase, especially as medical studies become more intricate and technology-oriented. PIs who adapt to these changes and enhance their skill sets will be well-positioned to lead advancements in medical research.
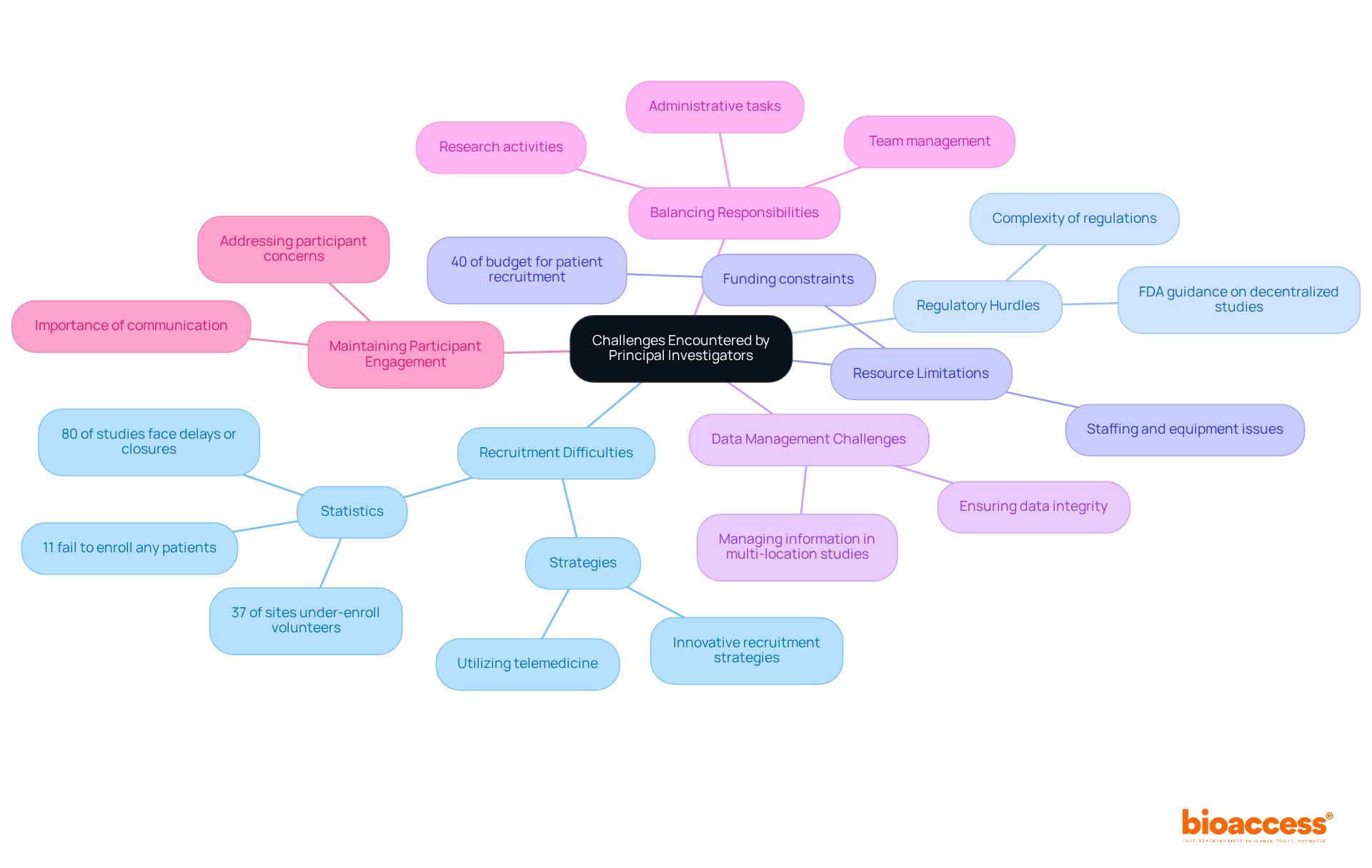
Lead Researchers (PIs) encounter numerous obstacles during the pi clinical trial process, which significantly impacts the success of their studies. Key challenges include:
Recruitment Difficulties: Attracting and retaining participants remains a persistent challenge, particularly for studies with stringent eligibility criteria or complex protocols. Approximately 80% of clinical studies face delays or closures due to recruitment issues, with 37% of sites under-enrolling volunteers and 11% failing to enroll any patients. This underscores the critical need for innovative recruitment strategies.
Regulatory Hurdles: Navigating the intricate regulatory landscape can be both time-consuming and complex. PIs must stay vigilant regarding evolving regulations and compliance requirements, which can vary significantly across regions. The FDA's recent guidance on decentralized studies emphasizes the importance of adapting to regulatory modifications to enhance patient access and involvement.
Resource Limitations: PIs frequently encounter constraints related to funding, staffing, and equipment, which can impede the execution of studies. The estimated cost of patient recruitment alone constitutes about 40% of the total budget, approximately $1.89 billion annually, highlighting the financial pressures faced by research teams.
Data Management Challenges: Ensuring data integrity while managing substantial amounts of information can be daunting, especially in multi-location studies. Effective data management strategies are essential for maintaining the quality and reliability of test results.
Balancing Responsibilities: PIs must navigate numerous responsibilities, including administrative tasks, team management, and research activities. This multifaceted role can lead to burnout, particularly when under pressure to meet recruitment targets and adhere to study timelines.
Maintaining Participant Engagement: Keeping participants engaged and motivated throughout the study is crucial for data quality and retention. Methods such as consistent communication and addressing participant concerns can significantly enhance retention rates, as research indicates that a positive patient experience increases the likelihood of participants completing the study.
By understanding these challenges, PIs can formulate targeted strategies to improve recruitment and retention, ultimately enhancing the success rates of the pi clinical trial.
The role of the Principal Investigator (PI) in clinical trials is undeniably crucial, serving as the backbone of research studies and ensuring that they are conducted ethically and effectively. This position demands not only a deep understanding of medical and scientific principles but also a commitment to participant safety and regulatory compliance. As the landscape of clinical research evolves, PIs must adapt to new technologies and methodologies to maintain their pivotal role in advancing medical knowledge.
Throughout the article, key responsibilities of PIs were highlighted, including:
The challenges faced by PIs, such as recruitment difficulties and regulatory hurdles, underscore the complexities inherent in clinical trials. Furthermore, the necessity for strong leadership, effective communication, and problem-solving skills emerged as vital attributes for PIs, reinforcing the need for well-rounded professionals in this field.
In conclusion, the significance of Principal Investigators in clinical research cannot be overstated. As they navigate the intricate landscape of medical studies, their leadership and expertise are essential for fostering innovation and ensuring the ethical conduct of trials. Embracing the challenges and responsibilities that come with this role is imperative for those aspiring to make meaningful contributions to the advancement of healthcare. The future of clinical research relies on the dedication and skill of PIs, making their role more important than ever in the quest for new treatments and improved patient outcomes.
What is the role of a Principal Investigator (PI) in clinical trials?
The Principal Investigator (PI) serves as the primary authority at a specific site in clinical trials, overseeing the study protocol, including preparation, execution, and management, while ensuring adherence to regulatory standards and ethical guidelines.
What qualifications do Principal Investigators typically have?
Principal Investigators are usually certified medical experts or investigators with the necessary expertise to supervise the study team and manage all aspects of the clinical trial.
What responsibilities does a PI have during a clinical trial?
A PI is responsible for participant recruitment, data collection, analysis, reporting, and maintaining study integrity while safeguarding participant welfare throughout the investigation.
How have the responsibilities of PIs changed with the transition towards digital transformation?
As we approach 2025, PIs are now expected to integrate advanced technologies, such as remote monitoring tools and electronic health records, into their workflows, enhancing data precision and streamlining participant engagement.
What impact do effective PIs have on clinical trial outcomes?
Effective PIs significantly improve research outcomes by managing multidisciplinary teams, maintaining clear communication with sponsors and regulatory bodies, and ensuring efficiency and compliance throughout the trial.
What ethical responsibilities do PIs have in clinical trials?
PIs are tasked with securing Institutional Review Board (IRB) approvals, monitoring adverse events, and ensuring informed consent, which are essential for preserving participant trust and regulatory compliance.
Why are PIs considered the cornerstone of medical studies?
PIs are instrumental in advancing medical knowledge and developing innovative treatments through their leadership in clinical trials, ensuring ethical conduct and the generation of high-quality data.