


The role of a US FDA agent for medtech innovators is critical, serving as the essential link between the FDA and international medical device manufacturers. This ensures compliance with U.S. regulations while facilitating effective communication.
Appointing a US FDA agent is not merely a regulatory requirement; it is a strategic necessity for foreign manufacturers. This appointment enables them to navigate the complexities of U.S. market entry, mitigate risks, and build credibility with the FDA.
Navigating the complex landscape of medical device regulation presents a formidable challenge for international manufacturers seeking entry into the U.S. market. Central to this endeavor is the role of a US FDA agent—an essential intermediary that not only ensures compliance with stringent regulations but also fosters effective communication between the FDA and foreign establishments. As the stakes escalate for medtech innovators, one pivotal question emerges: how can the appointment of the right US FDA agent significantly enhance the pathway to market success? Furthermore, what critical criteria must be considered in this essential selection process?
A US FDA agent acts as a vital link between the U.S. Food and Drug Administration (FDA) and international medical device manufacturers. This US FDA agent plays a crucial role in facilitating effective communication regarding compliance issues, enabling the FDA to engage with foreign establishments about their products. For businesses lacking a physical presence in the United States, the US FDA agent's role becomes even more significant, as they assist in navigating the complex legal framework and ensuring adherence to U.S. laws.
To effectively fulfill this role, the representative must either reside in the U.S. or maintain a business address there, guaranteeing their availability for FDA inquiries and communications. This arrangement not only streamlines the regulatory process but also enhances the manufacturer's capacity to respond promptly to any FDA requests, thereby fostering a smoother path to market entry.

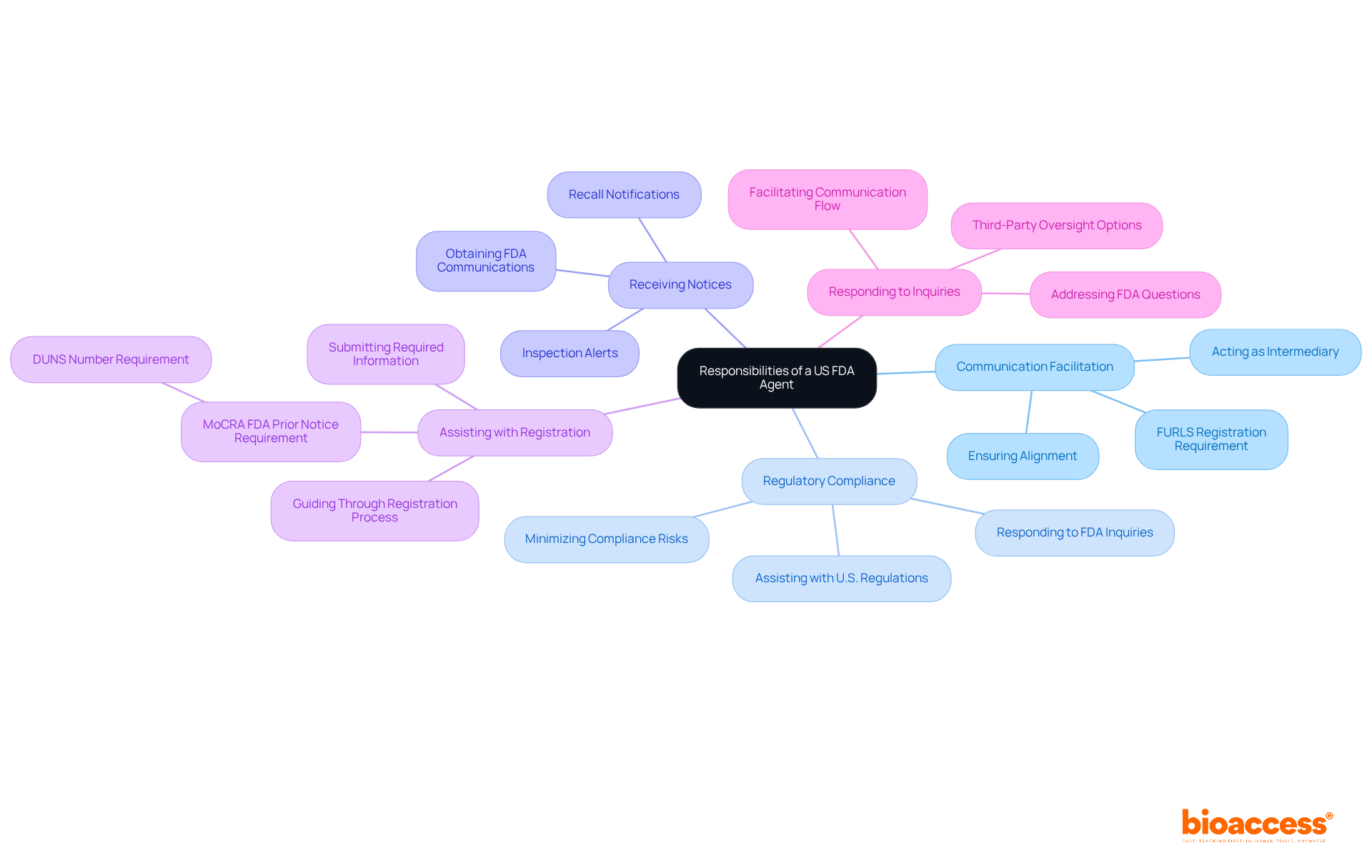
The responsibilities of a US FDA Agent encompass several key functions essential for effective communication and compliance:
Communication Facilitation: Acting as the main intermediary, the representative conveys essential information between the FDA and the international manufacturer, ensuring that both parties are aligned. Notably, without a US FDA agent, establishment registration in the FDA's FURLS system will not be accepted, highlighting the significance of this role.
Regulatory Compliance: The representative plays a crucial role in assisting international establishments adhere to U.S. regulations by responding to inquiries and providing necessary documentation, thereby minimizing compliance risks. This involves responding to any inquiries or requests for information from the FDA concerning the international establishment's products.
Receiving Notices: The representative is responsible for obtaining all FDA communications, including inspection alerts, recalls, and other compliance actions, ensuring that the international manufacturer is quickly informed.
Assisting with Registration: They guide international manufacturers through the FDA registration process, ensuring that all required information is submitted accurately and within the designated timelines, which is vital for maintaining operational continuity. This process is particularly important given the new requirement for the MoCRA FDA Prior Notice, which includes a DUNS number for FDA registration.
Responding to Inquiries: The agent must address any questions or requests for information from the FDA regarding the international establishment's products, facilitating a smooth flow of communication and enhancing oversight transparency. Moreover, independent third-party oversight service providers can serve as a US FDA agent, ensuring confidentiality and professionalism, which may be advantageous for international manufacturers seeking alternative options.
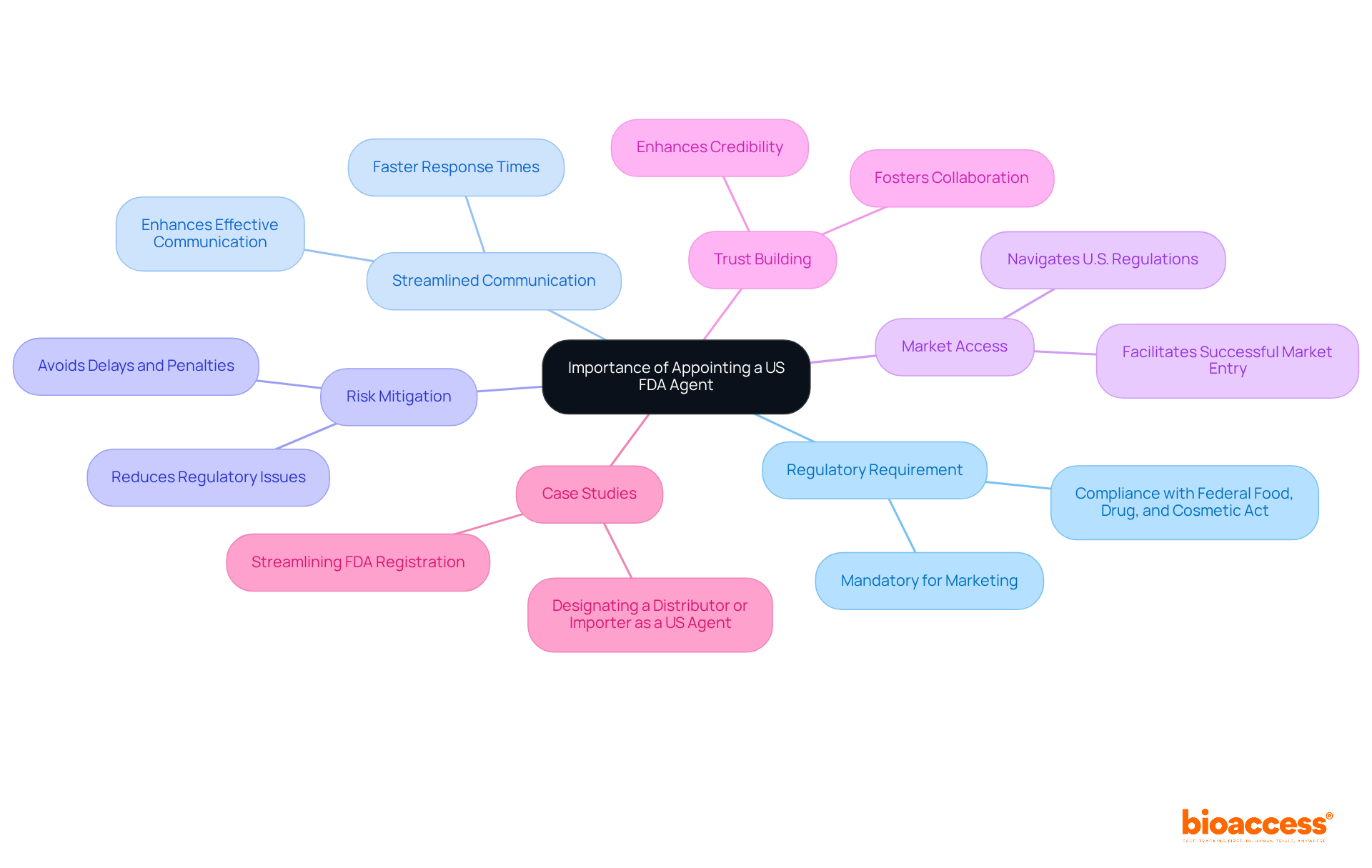
Appointing a US FDA Agent is crucial for several reasons:
Regulatory Requirement: For foreign manufacturers, having a US FDA Agent is a mandatory requirement for marketing medical devices in the U.S., as required by the Federal Food, Drug, and Cosmetic Act.
Streamlined Communication: The representative enhances effective communication with the FDA, which is essential for handling compliance inquiries and ensuring adherence. Having a US FDA agent ensures faster response times and full compliance with US regulations.
Risk Mitigation: By employing a knowledgeable representative, companies can reduce the risk of regulatory issues that could lead to delays or penalties. Without an FDA representative, businesses encounter risks such as delays, non-compliance penalties, and product rejection.
Market Access: The US FDA agent plays a pivotal role in assisting international companies navigate the complexities of U.S. regulations, ultimately aiding in successful market entry. The representative serves as a crucial connection between the manufacturer and the US FDA agent, facilitating communication to address regulatory inquiries and ensure compliance.
Trust Building: Appointing a dependable representative can enhance the credibility of an international manufacturer in the eyes of the FDA and other stakeholders, fostering trust and collaboration. Their responsibilities include handling FDA communication, assisting with facility registration, receiving and responding to FDA inquiries and notifications, and ensuring compliance with FDA regulations.
Case Studies: For instance, the 'Streamlining FDA Registration' case study illustrates how a representative from the FDA helps prevent delays and errors in the registration process, emphasizing the practical implications of appointing a US FDA representative.
In summary, the appointment of a US FDA agent is not merely a procedural formality; it is a strategic necessity for foreign manufacturers aiming to succeed in the U.S. market.
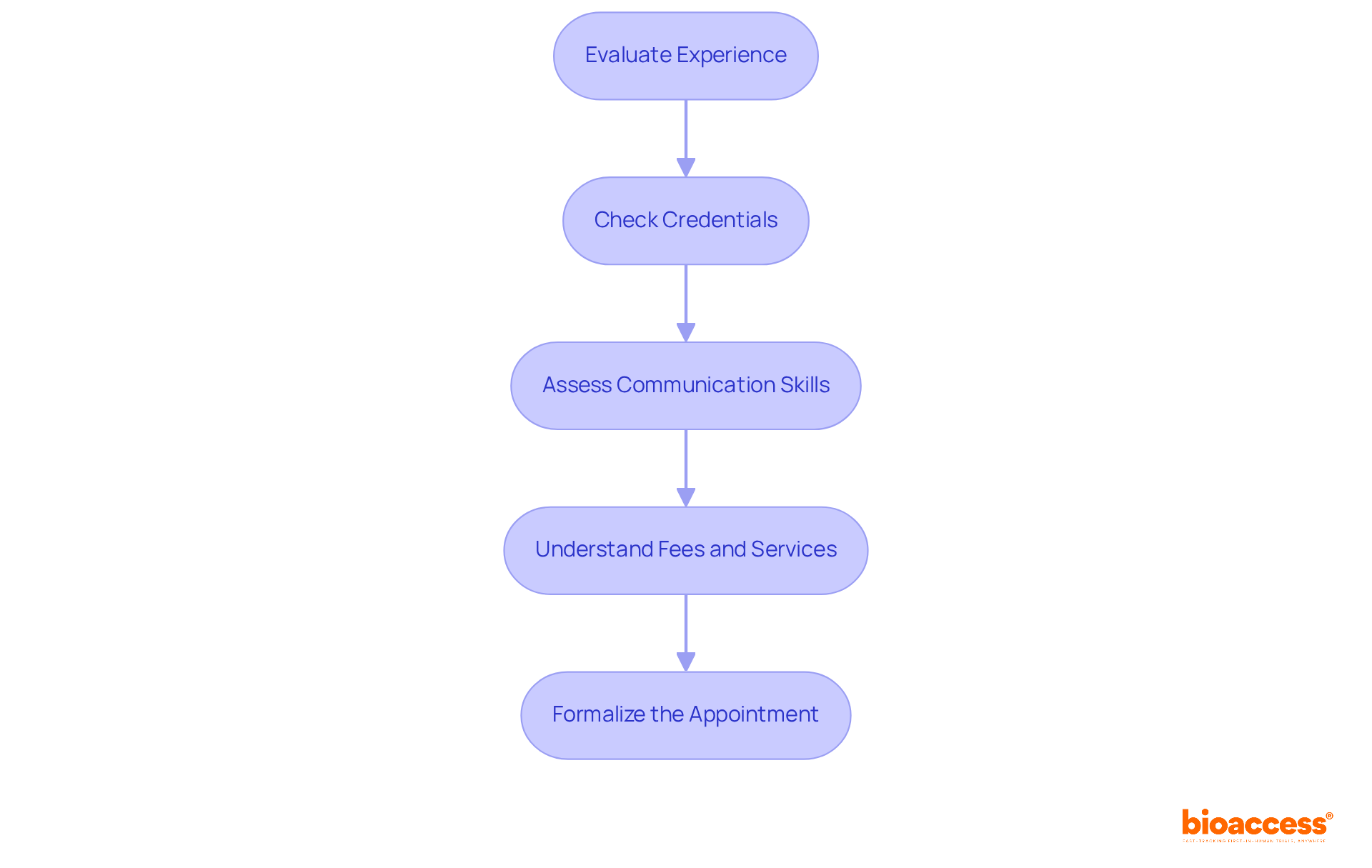
Selecting and designating a US FDA agent is a pivotal process that requires careful consideration to ensure compliance and effective communication with the FDA.
Evaluate Experience: It is crucial to prioritize agents who possess a robust background in compliance matters and a proven track record with FDA processes. Their expertise can significantly influence the success of your product's market entry. For instance, specialists like Ana Criado, Director of Compliance at Bioaccess, bring extensive experience in oversight roles and advising global companies, ensuring a comprehensive understanding of the FDA landscape. Additionally, Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, offers valuable insights into the regulatory environment.
Check Credentials: Confirming that the representative is a lawfully registered firm in the U.S. and possesses the necessary knowledge of medical device regulations is essential. This verification helps avoid complications during the registration process.
Assess Communication Skills: It is vital to choose a representative who excels in communication, as they will act as the primary liaison with the FDA. Effective communication is key for timely updates and addressing any regulatory inquiries.
Understand Fees and Services: Clarifying the fee structure and specific services offered by the representative ensures that their offerings align with your operational needs and budget.
Formalize the Appointment: Once you have selected an agent, formalize the appointment by submitting the required documentation to the FDA. Ensure that all information is accurate and up-to-date to prevent any delays or issues with compliance.
By adhering to these steps, Medtech innovators can forge a reliable partnership with a US FDA agent, thereby facilitating smoother interactions with regulatory authorities and enhancing the likelihood of successful product commercialization.
The role of a US FDA agent is indispensable for international medical device manufacturers seeking to navigate the complexities of the U.S. regulatory landscape. This agent serves as a crucial intermediary, ensuring seamless communication with the FDA and maintaining compliance with U.S. laws. Without a designated representative, foreign manufacturers face significant hurdles in establishing their products in the U.S. market, underscoring the strategic necessity of appointing a knowledgeable US FDA agent.
Key points throughout the article underscore the multifaceted responsibilities of a US FDA agent, including:
The importance of having a US FDA agent is further emphasized through discussions on risk mitigation, market access, and trust-building with the FDA. Additionally, practical guidance on selecting and designating an agent provides valuable insights for Medtech innovators aiming to enhance their operational success.
In essence, the appointment of a US FDA agent transcends a mere regulatory requirement; it represents a strategic move that can significantly impact a company's ability to successfully bring medical devices to market in the U.S. By understanding the vital role these agents play and following the outlined steps for selection, Medtech companies can better position themselves for compliance and growth in a competitive landscape. Embracing this partnership not only streamlines the path to market entry but also fosters a foundation of trust essential for long-term success in the medical technology sector.
What is a US FDA agent?
A US FDA agent is a representative that acts as a vital link between the U.S. Food and Drug Administration (FDA) and international medical device manufacturers, facilitating communication regarding compliance issues.
What is the purpose of a US FDA agent?
The purpose of a US FDA agent is to assist international manufacturers in navigating the U.S. legal framework and ensuring adherence to U.S. laws, particularly for those without a physical presence in the United States.
Why is the role of a US FDA agent important for businesses without a U.S. presence?
For businesses lacking a physical presence in the U.S., the US FDA agent's role is crucial as they help navigate complex legal requirements and ensure compliance with U.S. regulations, enhancing the manufacturer's ability to respond to FDA inquiries.
What are the requirements for a US FDA agent?
To effectively fulfill their role, a US FDA agent must either reside in the U.S. or maintain a business address there, ensuring availability for FDA inquiries and communications.
How does a US FDA agent facilitate the regulatory process?
A US FDA agent streamlines the regulatory process by enhancing communication between the FDA and manufacturers, allowing for prompt responses to FDA requests and fostering a smoother path to market entry.