


Chile has emerged as a prominent hub for clinical trials, offering a unique blend of robust healthcare infrastructure and regulatory support that attracts research initiatives from around the globe. The country's strategic advantages include access to a diverse patient population, lower operational costs compared to North America and Europe, and a regulatory environment that aligns with international standards. As Medtech startups seek to navigate the complexities of clinical research, organizations like Bioaccess® play a crucial role in streamlining processes and enhancing the efficiency of trial management.
This article delves into the multifaceted landscape of clinical trial services in Chile, exploring the benefits, regulatory framework, and effective patient recruitment strategies that collectively position the nation as an ideal destination for advancing medical innovation.
Trial services in Chile include a wide array of activities associated with the planning, management, and execution of medical studies. These services encompass:
Bioaccess®, a prominent contract study organization in Latin America, offers top-tier trial services, allowing Medtech startups to progress their medical devices effectively. With Chile's robust healthcare system and regulatory framework, combined with Bioaccess®'s expertise in navigating complex regulations and expediting study results, the country provides an ideal environment for trials.
The Chilean administration has created guidelines in line with global standards, promoting a favorable environment for medical studies. However, medical device startups often face significant challenges, including:
The existence of skilled study facilities and an increasing number of patients eager to take part in experiments improves the overall environment for service offerings in the nation. By collaborating with Bioaccess®, startups can utilize customized solutions that not only tackle these challenges but also simplify the route to successful evaluations, ultimately advancing their medical devices to market more swiftly.
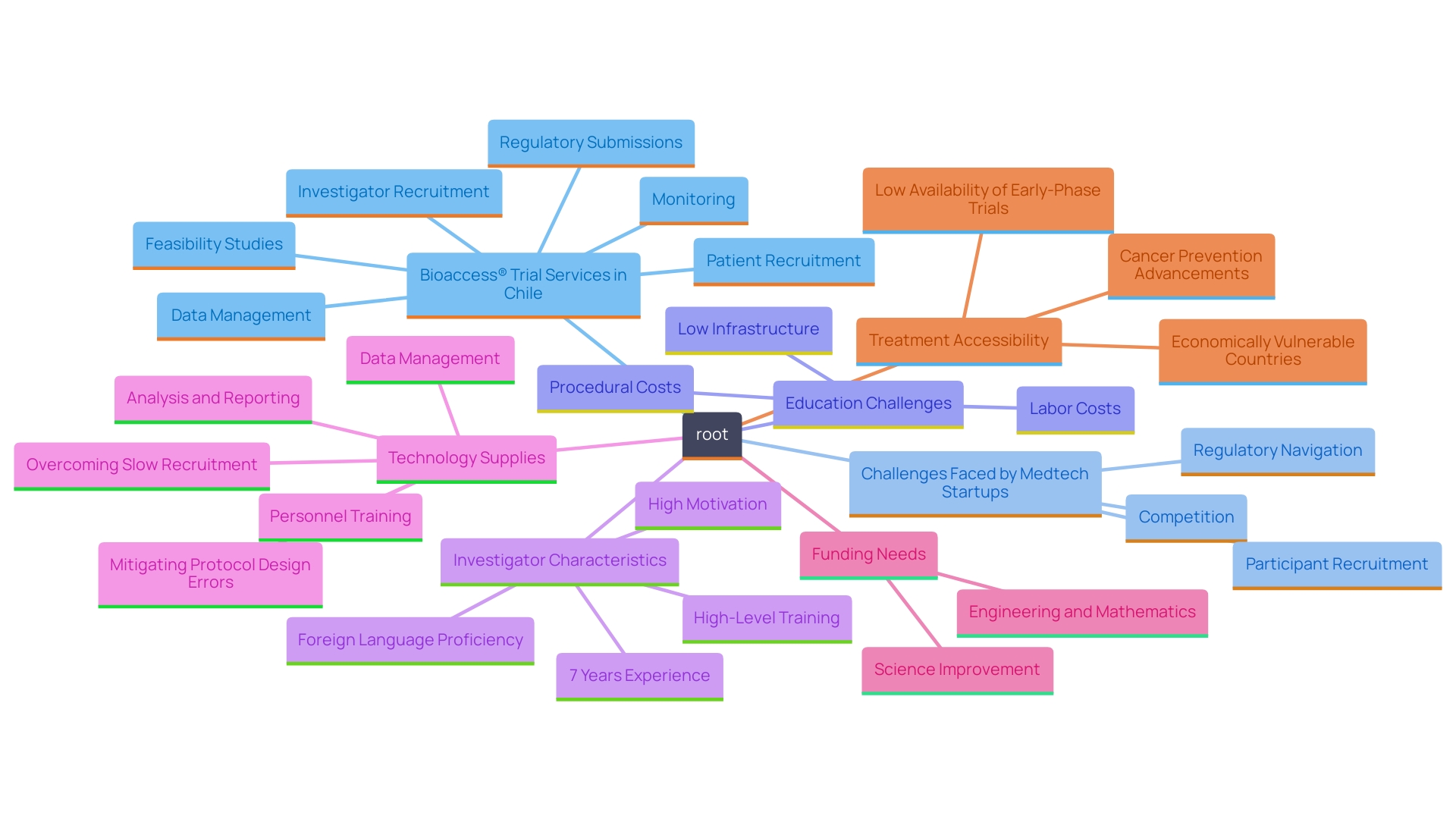
Carrying out clinical studies in Chile offers various benefits that can greatly improve research results. A primary benefit is access to a diverse group of individuals, which facilitates robust recruitment efforts and yields findings that are more generalized and applicable. This diversity is especially advantageous in contrast to North America, where participant populations may be less diverse, possibly restricting the relevance of study outcomes. Additionally, the high standard of care within Chile's healthcare system contributes to improved patient compliance and retention throughout the study duration.
Furthermore, operational expenses in Chile are significantly lower than in North America and Europe, offering a cost-efficient solution for pharmaceutical firms and organizations. For example, conducting a clinical study in Chile can cost up to 40% less than in the United States, enabling more extensive research within the same budget.
The regulatory framework in Chile is also favorable, featuring streamlined processes that expedite study approvals and accelerate the time-to-market for new treatments. This efficiency is further backed by increasing interest and investment from both local and international sponsors, highlighting Chile's market potential and dedication to advancing medical studies.
Nuestras capacidades de servicio incluyen:
This comprehensive approach ensures high-quality health outcomes, as evidenced by the inclusion of uniprofessional interventions in ten programs, demonstrating the country's proactive stance on specialized care.
As one specialist in respiratory health observes, 'Reception in the Acute Respiratory Diseases patient area referred from the health network due to an acute respiratory or exacerbation of a chronic respiratory condition, to access a visit with the physical therapist within the deadlines established Explicit Health Guarantees for patients,' demonstrates the rigorous standards of care maintained in healthcare environments, which is essential for success.
A case study on the necessity for clearer health policy translation in Chile further illustrates the country's dedication to improving healthcare practices. The study concluded that translating health policies into actionable program documents fosters person-centered care and interprofessional collaboration, ultimately enhancing the quality of care in primary settings. This dedication to enhancing healthcare methods emphasizes the benefits of carrying out studies in Chile, rendering it an appealing location for investigative projects while also positively impacting local economies through job creation and economic development.
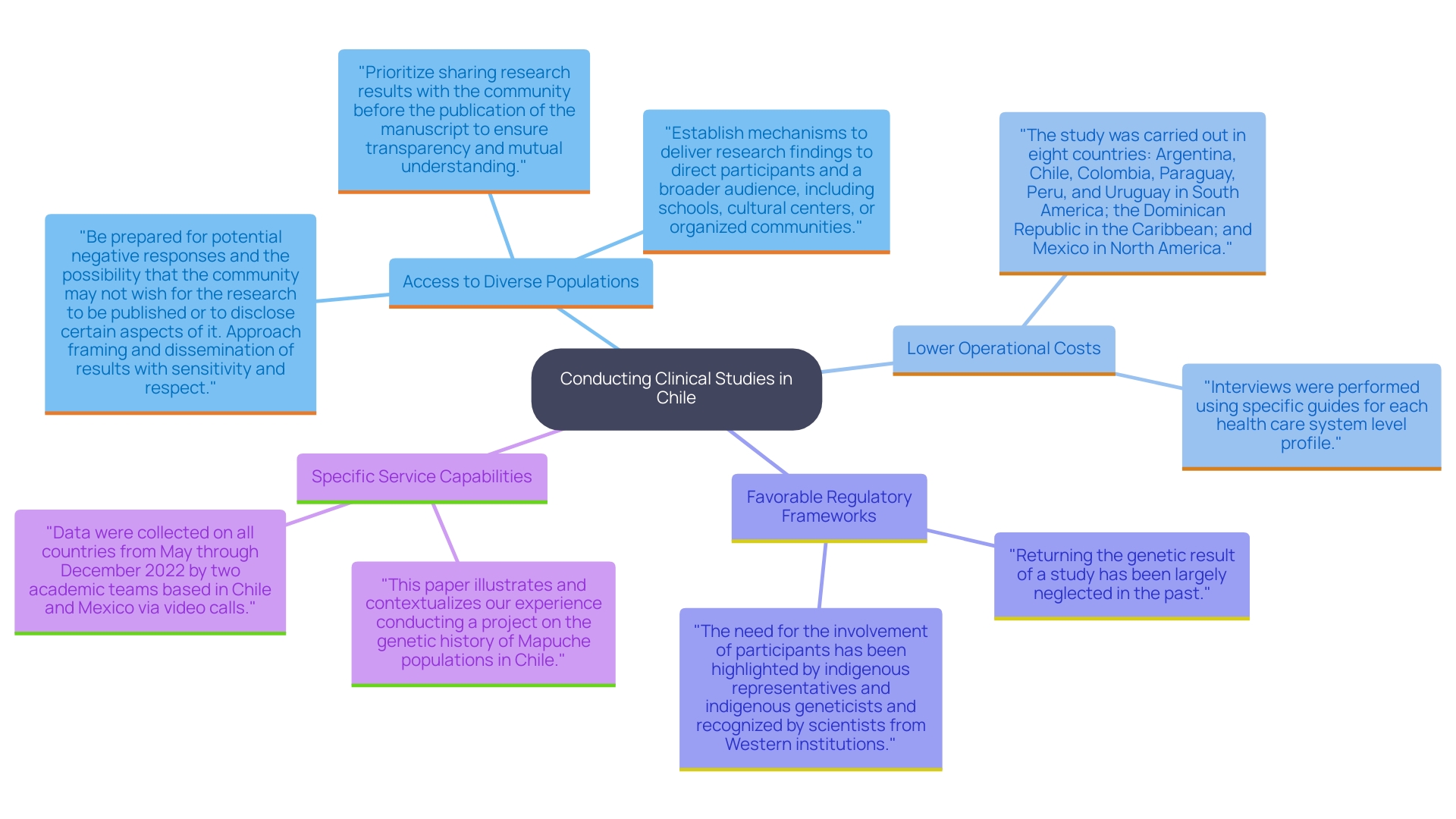
The regulatory environment for research studies in Chile is carefully overseen by the Instituto de Salud Publica (ISP) and the Ministry of Health. These entities are pivotal in enforcing regulations that uphold patient safety, data integrity, and ethical standards throughout the research process. Before starting a research study, sponsors must submit a comprehensive application including study protocols, informed consent documents, and safety data to obtain approval. The ISP also performs rigorous inspections to ensure adherence to Good Clinical Practice (GCP) guidelines, which are aligned with international benchmarks.
In this regulatory context, bioaccess® provides a complete range of research management services. These services encompass:
By tackling all facets of bioassays, bioaccess® greatly improves the efficiency and effectiveness of the study process.
Ethics committees play an integral role in this framework, tasked with reviewing studies to safeguard participant rights. Katherine Ruiz, a Regulatory Affairs expert at bioaccess®, is instrumental in navigating these regulatory complexities for medical devices and in vitro diagnostics in Colombia. Her expertise not only aids in compliance but also fosters an environment conducive to innovation in Medtech.
This comprehensive regulatory structure instills confidence among stakeholders and promotes a transparent research environment prioritizing patient welfare. Under the leadership of experts like Amanshe Slaney, who has significantly enhanced BioIVT's compliance with regulatory standards since 2014, the importance of continuous improvement and accurate interpretation of regulations is evident. Slaney emphasizes, 'My team and I are dedicated to continuing this trajectory of growth and high standards.' Her efforts have resulted in a betterment of the company's compliance rates, with BioIVT achieving a 95% success rate in securing research approvals in the past year.
Recent updates from the ISP reflect ongoing efforts to refine the regulatory framework. For example, the ISP has launched a new initiative designed to accelerate the review process for research applications, which is anticipated to decrease approval times by as much as 20% in the upcoming year. Such initiatives strengthen Chile's dedication to upholding high standards in research studies, which not only aids researchers and individuals but also enhances local economies. The ripple effects include job creation, economic growth, and improved healthcare outcomes, demonstrating the broader impact of research studies in enhancing community well-being.
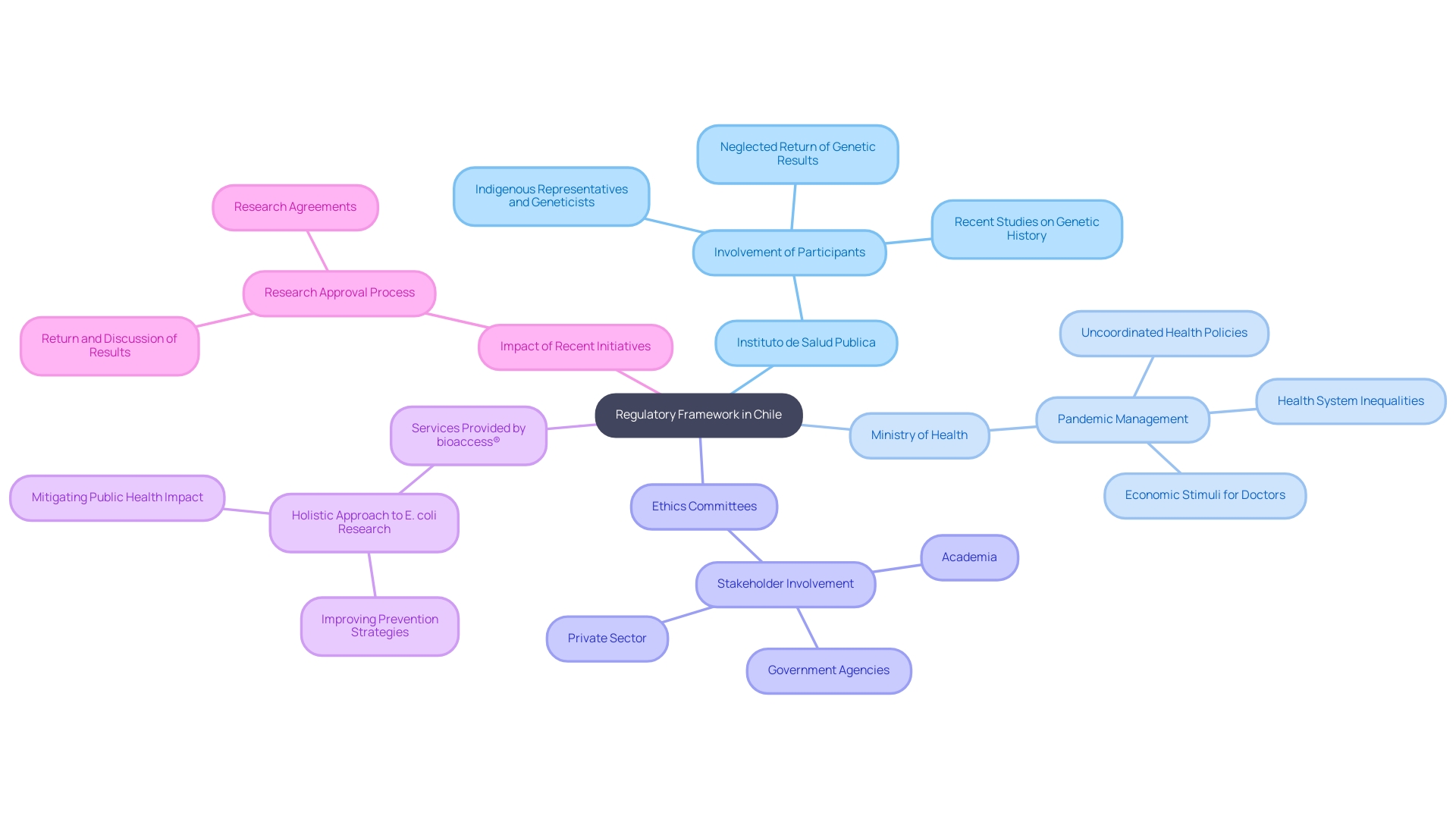
Successful participant recruitment is essential to the success of clinical studies in Chile. Strategies may involve:
Working together with advocacy organizations can also improve recruitment efforts, especially for studies involving particular diseases. Additionally, ensuring that the informed consent process is clear and accessible helps build trust with potential participants, making them more likely to enroll.
By employing a multifaceted approach to patient recruitment, researchers can enhance participation rates and ensure a diverse study population, ultimately leading to more reliable and generalizable findings.
Our comprehensive trial management services—feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—are designed to support successful trials. Notably, Clinical Leader has recognized the growing importance of clinical research in Latin America, particularly in Colombia, which adds credibility to our services and highlights the region's potential for impactful studies.
Chile's emergence as a key player in the clinical trial landscape underscores its unique advantages, making it an attractive destination for Medtech startups. The country boasts a robust healthcare infrastructure, a diverse patient population, and a regulatory framework that aligns with international standards, all of which facilitate efficient research processes. With organizations like Bioaccess® providing essential support in navigating the complexities of clinical trials, startups can focus on their core innovations while ensuring compliance and effective trial management.
The benefits of conducting clinical trials in Chile are manifold. From lower operational costs to high standards of patient care, the environment is conducive to generating reliable, generalizable findings. This is further enhanced by the commitment of the Chilean government to streamline regulatory processes, thereby expediting the time-to-market for new treatments.
As demonstrated through various strategies for patient recruitment and engagement, the potential for successful trial outcomes is significantly increased, reinforcing Chile's position as a leading hub for clinical research.
In conclusion, the combination of a supportive regulatory framework, a diverse patient base, and efficient trial management services creates a fertile ground for advancing medical innovation in Chile. By leveraging these advantages, Medtech startups can not only accelerate their research initiatives but also contribute positively to the local economy and healthcare landscape. As the global demand for clinical trials continues to grow, Chile stands ready to meet the challenge, fostering an environment that prioritizes patient welfare and scientific advancement.
What services are included in trial services in Chile?
Trial services in Chile include feasibility and selection of study sites, investigator selection, patient recruitment, regulatory submissions, monitoring, and data management.
How does Bioaccess® support Medtech startups in Chile?
Bioaccess® offers top-tier trial services that help Medtech startups navigate complex regulations and expedite study results, facilitating the effective progress of their medical devices.
What challenges do medical device startups face in Chile?
Startups often face challenges such as navigating convoluted regulatory processes, competing against established players, and recruiting suitable participants.
What advantages does Chile offer for conducting clinical studies?
Chile provides access to a diverse population for recruitment, a high standard of care that improves patient compliance, significantly lower operational costs compared to North America, and a favorable regulatory framework that accelerates study approvals.
How much can conducting a clinical study in Chile cost compared to the United States?
Conducting a clinical study in Chile can cost up to 40% less than in the United States.
What are the roles of the Instituto de Salud Publica (ISP) and the Ministry of Health in Chile's regulatory environment?
The ISP and the Ministry of Health oversee the regulatory environment, ensuring patient safety, data integrity, and ethical standards throughout the research process.
What is required from sponsors to start a research study in Chile?
Sponsors must submit a comprehensive application, including study protocols, informed consent documents, and safety data, to obtain approval.
What recent initiatives have been introduced by the ISP to improve the regulatory process?
The ISP has launched initiatives aimed at accelerating the review process for research applications, potentially decreasing approval times by up to 20%.
What strategies can researchers use for successful participant recruitment in Chile?
Strategies include collaborating with local healthcare providers, employing social media campaigns, organizing informational sessions, and working with advocacy organizations.
How does participant diversity benefit clinical studies in Chile?
A diverse participant population enhances recruitment efforts and yields findings that are more generalized and applicable, improving the relevance of study outcomes.