


Clinical Data Management Systems (CDMS) are pivotal in clinical research, providing a structured framework for the collection, storage, and analysis of trial data. This structured approach is essential for ensuring precision and regulatory compliance in clinical trials. CDMS significantly enhances data integrity, reduces human error, and facilitates real-time monitoring, which collectively leads to more efficient clinical trials. Consequently, this efficiency fosters informed decision-making in the development of new medical therapies, underscoring the critical role of CDMS in advancing healthcare innovation.
In the intricate landscape of clinical research, the integrity and management of data are not just important—they are critical to the success of medical advancements. Clinical Data Management Systems (CDMS) provide the essential framework for collecting, storing, and analyzing trial data, transforming raw information into meaningful insights that drive decision-making. However, as the complexity of clinical trials increases, researchers face a pressing question: how can these systems not only maintain data integrity but also enhance overall efficiency? This article delves into the vital role of CDMS, exploring its evolution, key functionalities, and the significant impact it has on the future of clinical research.
Clinical Trial Management Systems (CTMS) are specialized software applications designed to oversee and optimize the collection, storage, and analysis of trial information. These systems play a crucial role in the effective management of data generated during medical research, ensuring precision, security, and compliance with regulatory standards. Furthermore, understanding what is a CDMS is integral in transforming raw data into actionable insights, which are essential for informed decision-making in the development of new medical therapies and technologies.
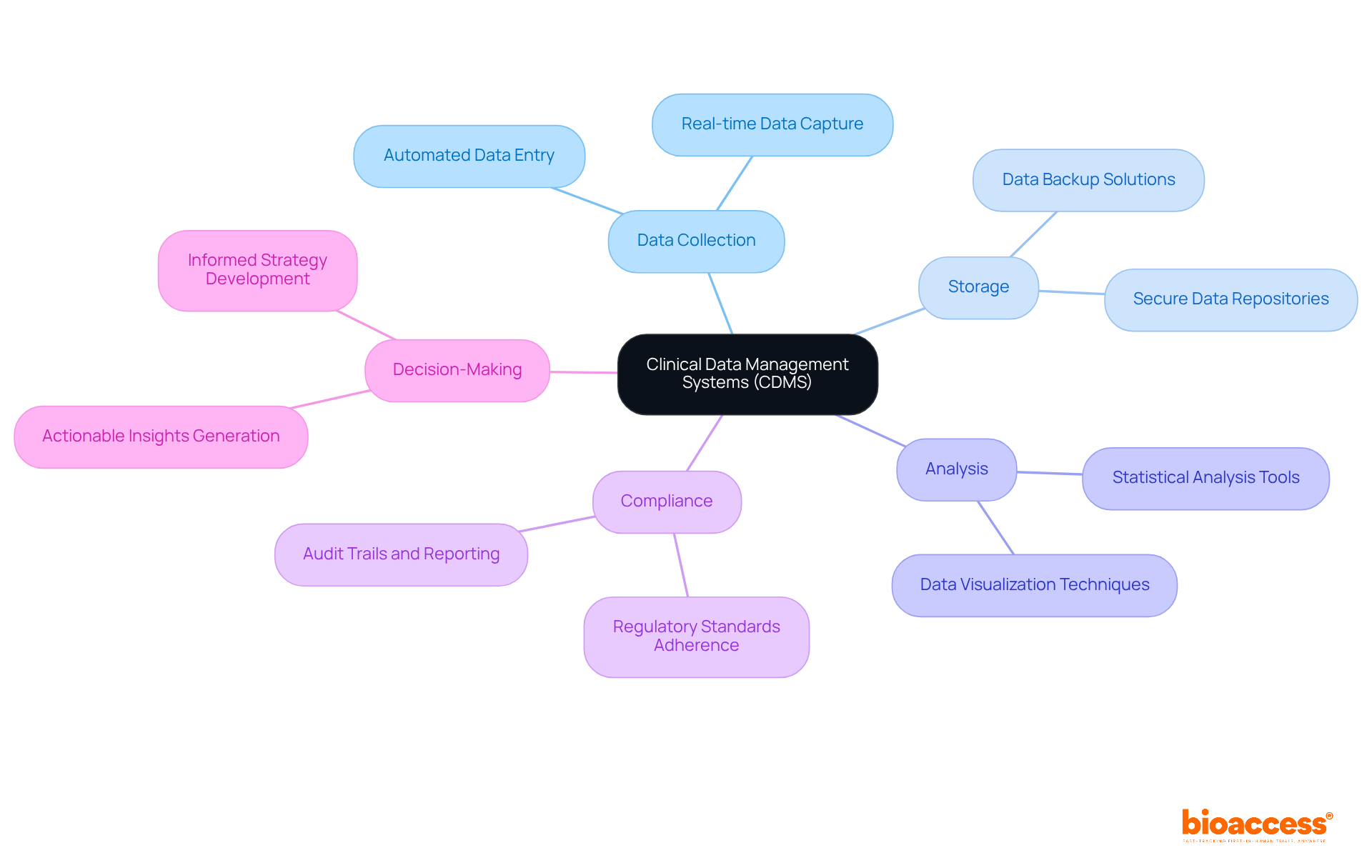
In the realm of medical research, the integrity and precision of information are paramount. What is a CDMS? It refers to clinical data management systems that provide a structured framework for overseeing clinical research records, which is vital for regulatory compliance and the effective approval of new treatments.
By automating the processes of information collection and management, these systems significantly reduce the risk of human error, enhance quality, and facilitate faster decision-making. Furthermore, they enable real-time monitoring of information, allowing researchers to swiftly identify trends and issues, thereby improving overall study efficiency.
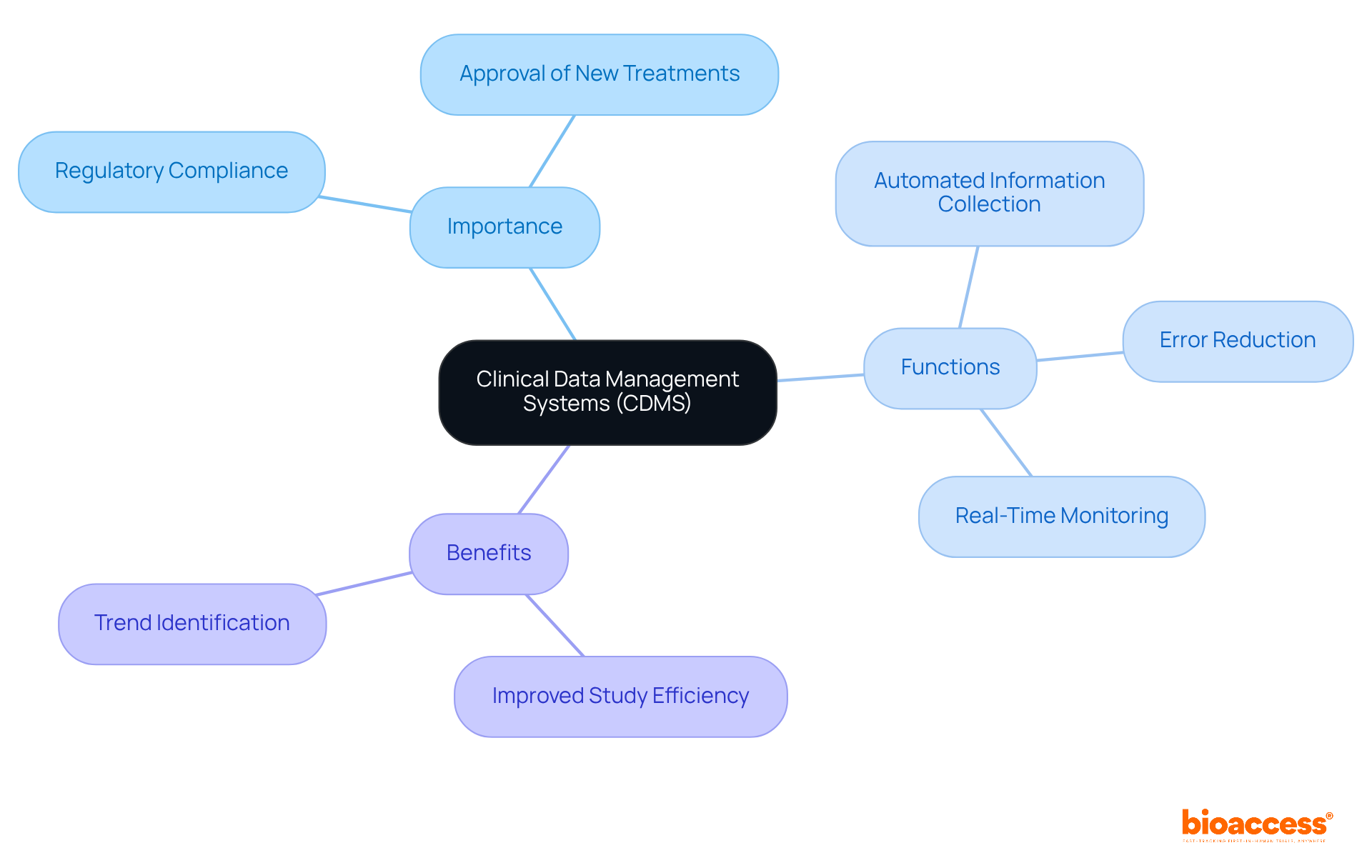
The evolution of Clinical Information Management Systems (CIMS) is rooted in the early days of research, where information was predominantly collected and managed through paper-based methods. As clinical trials became increasingly complex and the volume of information surged, the demand for more efficient information management solutions emerged.
The advent of electronic data capture (EDC) systems in the late 1990s represented a pivotal shift, enabling real-time data entry and monitoring. This transformation was crucial, particularly considering that individuals spend 60% to 80% of their time searching for information, leading to significant productivity losses.
Over the years, content management systems have advanced, incorporating cutting-edge technologies such as cloud computing, artificial intelligence, and machine learning to enhance information processing capabilities and improve user experience.
As Peter Sondergaard aptly noted, "Information is the oil of the 21st century, and analytics is the combustion engine," underscoring the vital role of information in clinical research. Furthermore, the human aspect of analytics remains essential for effectively interpreting data, ensuring that insights derived from content management systems are actionable and relevant.
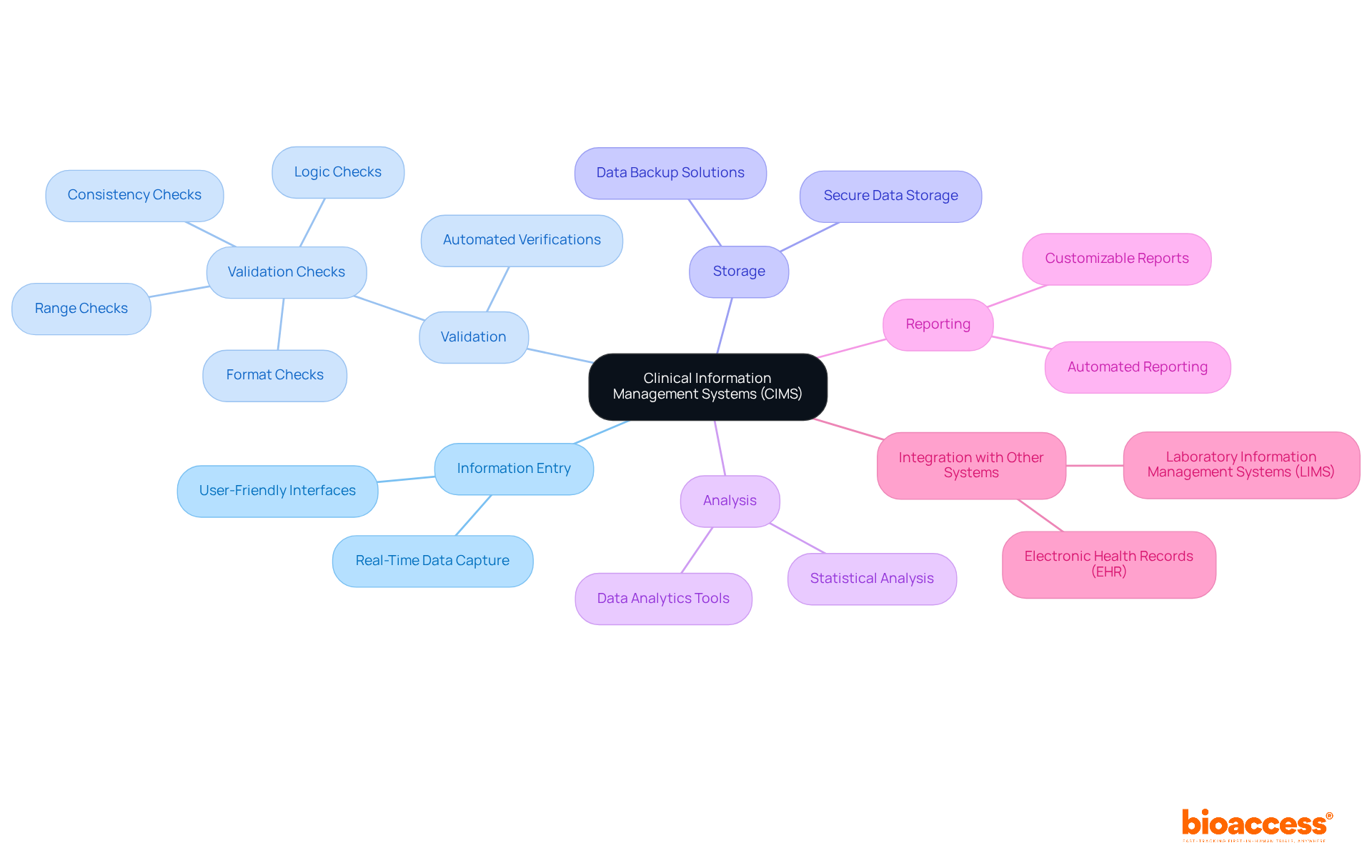
Key elements of Clinical Information Management Systems (CIMS) encompass information entry and validation, information storage, information analysis, and reporting functionalities. These systems are designed with user-friendly interfaces that facilitate efficient information input while adhering to regulatory standards. Validation tools are crucial to clinical data management systems, systematically identifying discrepancies and errors, thereby significantly enhancing information integrity. For instance, automated verifications within the system can promptly highlight discrepancies, ensuring that information remains precise and dependable throughout the process.
Moreover, robust reporting capabilities empower researchers to generate critical insights and summaries essential for informed decision-making. The integration of Clinical Data Management Systems (CDMS) with other systems, such as electronic health records (EHR) and laboratory information management systems (LIMS), illustrates what is a CDMS by streamlining information flow and fostering a cohesive and efficient clinical trial environment. Regular audits are instrumental in identifying and rectifying issues in the validation process, ensuring ongoing compliance and integrity. Additionally, establishing a Validation Plan that delineates objectives focused on accuracy, completeness, and consistency is vital for maintaining high quality.
Statistics reveal that inadequate data validation can result in error rates ranging from 2 to 2,784 errors per 10,000 fields, underscoring the necessity of implementing effective validation protocols to uphold high data quality and regulatory compliance.
Clinical Data Management Systems (CDMS) are indispensable in the realm of clinical research, acting as the backbone for the management of extensive data generated during trials. By automating data collection, storage, and analysis, these systems enhance the quality and integrity of information while ensuring compliance with regulatory standards. This leads to more efficient and informed decision-making in the development of new therapies.
The historical evolution of CDMS is noteworthy, transitioning from early paper-based methods to the advanced technologies we see today, such as cloud computing and artificial intelligence. Key functionalities—data entry validation, storage, analysis, and reporting—are essential for maintaining high data quality and regulatory compliance. Furthermore, the integration of CDMS with other systems streamlines the clinical trial process, showcasing its multifaceted contributions to research efficiency.
As the demand for reliable and precise data escalates, the importance of CDMS in clinical research becomes increasingly evident. Embracing these systems is not just a choice; it is a necessity for researchers who seek to enhance the integrity of their studies and expedite the approval of new medical breakthroughs. By prioritizing the implementation of robust CDMS, the clinical research community can ensure that the insights derived from their data lead to meaningful advancements in healthcare.
What are Clinical Data Management Systems (CDMS)?
Clinical Data Management Systems (CDMS) are specialized software applications designed to oversee and optimize the collection, storage, and analysis of information generated during clinical trials.
What is the primary purpose of a CDMS?
The primary purpose of a CDMS is to effectively manage data generated during medical research, ensuring precision, security, and compliance with regulatory standards.
How do CDMS contribute to medical research?
CDMS contribute to medical research by transforming raw data into actionable insights, which are essential for informed decision-making in the development of new medical therapies and technologies.