


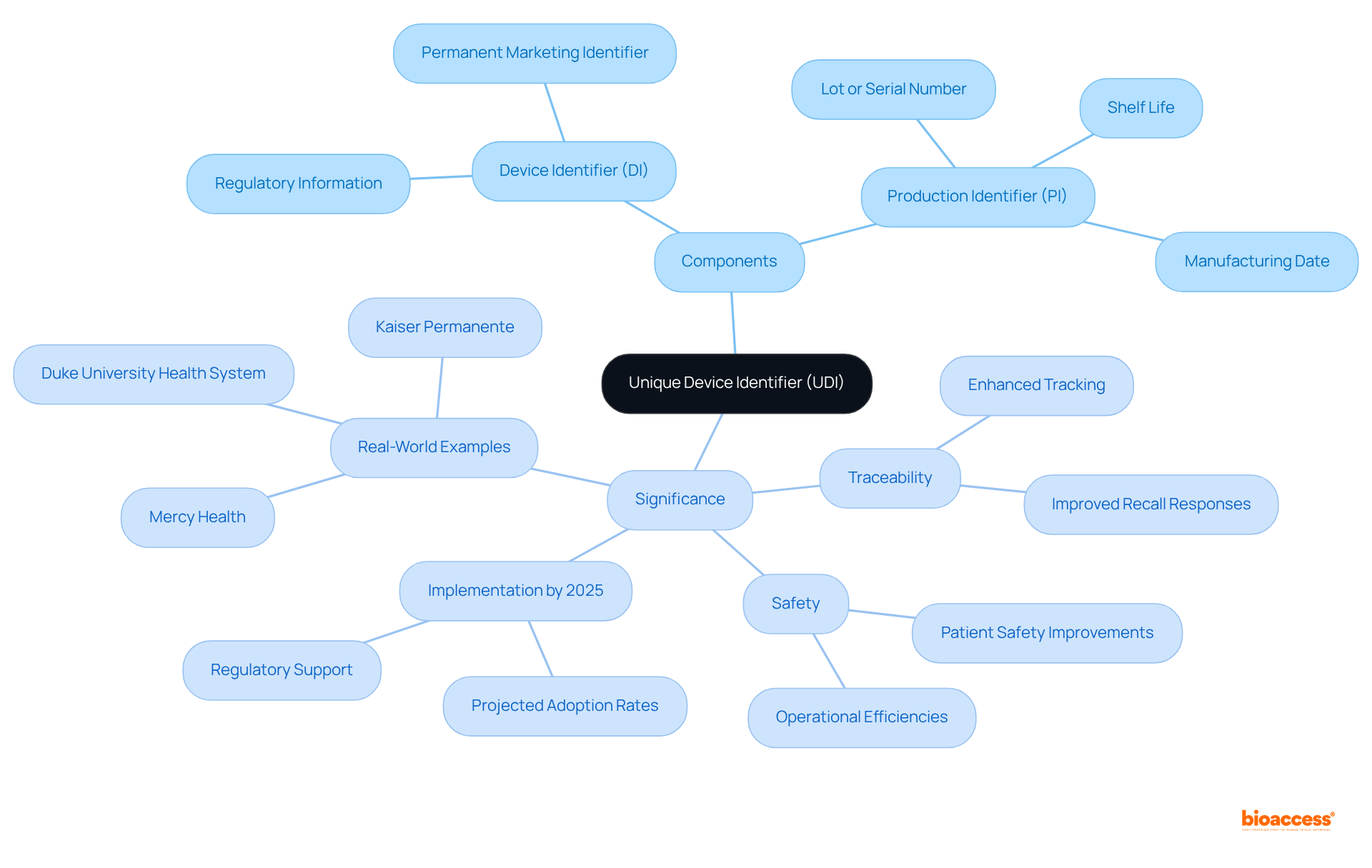
The Unique Device Identifier (UDI) is a standardized code assigned to medical devices, ensuring their traceability and enhancing safety in healthcare settings. This critical mechanism plays an essential role in improving patient safety and compliance by providing precise identification of devices. Research indicates that the implementation of UDI has significantly reduced medical errors, thereby improving overall healthcare outcomes. The importance of UDI cannot be overstated, as it directly contributes to the integrity of clinical practices and patient welfare.
The healthcare landscape is increasingly reliant on technology, making the safety and traceability of medical devices more critical than ever. Unique Device Identification (UDI) systems have emerged as a pivotal solution, offering a standardized method to track and manage medical instruments throughout their lifecycle. As UDI implementation becomes more widespread, questions arise regarding its true impact on patient safety and operational efficiency. How can a deeper understanding of UDI transform healthcare practices and enhance outcomes for both patients and providers alike?
What is a UDI? It is a standardized numeric or alphanumeric code assigned to medical instruments, designed to uniquely identify them throughout their lifecycle. This system comprises two main components:
This framework is crucial for guaranteeing traceability and safety in healthcare environments, which involves understanding what is a UDI for the equipment utilized in patient care. By 2025, it is projected that approximately 80% of medical instruments will incorporate UDI, which raises the question of what is a UDI, underscoring its growing significance in enhancing safety for individuals and optimizing supply chain management.
The FDA's commitment to UDI implementation emphasizes what is a UDI and its potential to improve health outcomes and operational efficiencies, as noted by experts like Alberto Jarrin Lopez. Real-world examples, such as the successful UDI implementation at Duke University Health System, demonstrate substantial improvements in recall responses and overall patient safety, which illustrates what is a UDI's essential role in contemporary healthcare.
Nevertheless, challenges persist, including the necessity for stronger policy support and increased awareness of what is a UDI and its benefits among healthcare leaders. The AccessGUDID database is pivotal in this system, providing a centralized resource for identification and tracking, thereby further enhancing clinical practice and illustrating what is a UDI.
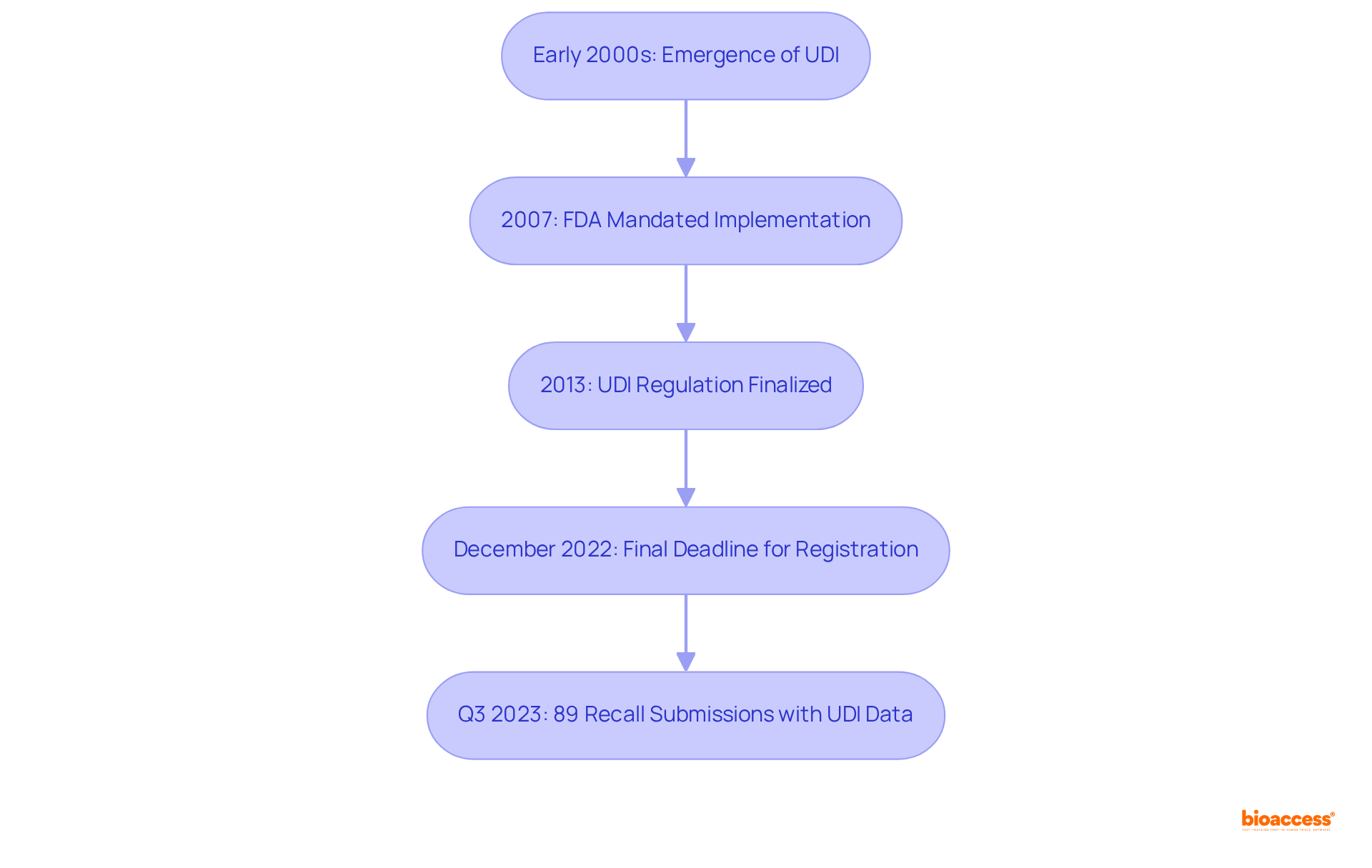
The concept of Unique Device Identification (UDI), or what is a udi, emerged in the early 2000s, driven by the pressing need for improved safety and traceability of healthcare instruments. In 2007, U.S. Congress mandated the FDA to implement a system for what is a UDI as part of the Food and Drug Administration Amendments Act. By 2013, the FDA finalized the UDI regulation, which clarified what is a UDI and required most medical products to feature a UDI on their labels and packaging. This evolution reflects a growing recognition of the importance of equipment traceability in enhancing safety for patients and streamlining regulatory processes. Notably, the final deadline for mandatory registration, which raises the question of what is a UDI, was December 2022, marking a pivotal moment in the UDI implementation timeline. Since the UDI system's inception, the GUDID database has grown to include over 4 million unique item records, surpassing 1 million entries in September 2016. Furthermore, the percentage of recall submissions for equipment that incorporated UDI data reached 89% in Q3 2023, underscoring the system's role in improving recall processes and ensuring the correct products are utilized with the appropriate patients. As the UDI system continues to evolve, it serves to illustrate what is a udi as a cornerstone in the ongoing efforts to enhance the safety and efficacy of medical equipment in healthcare.
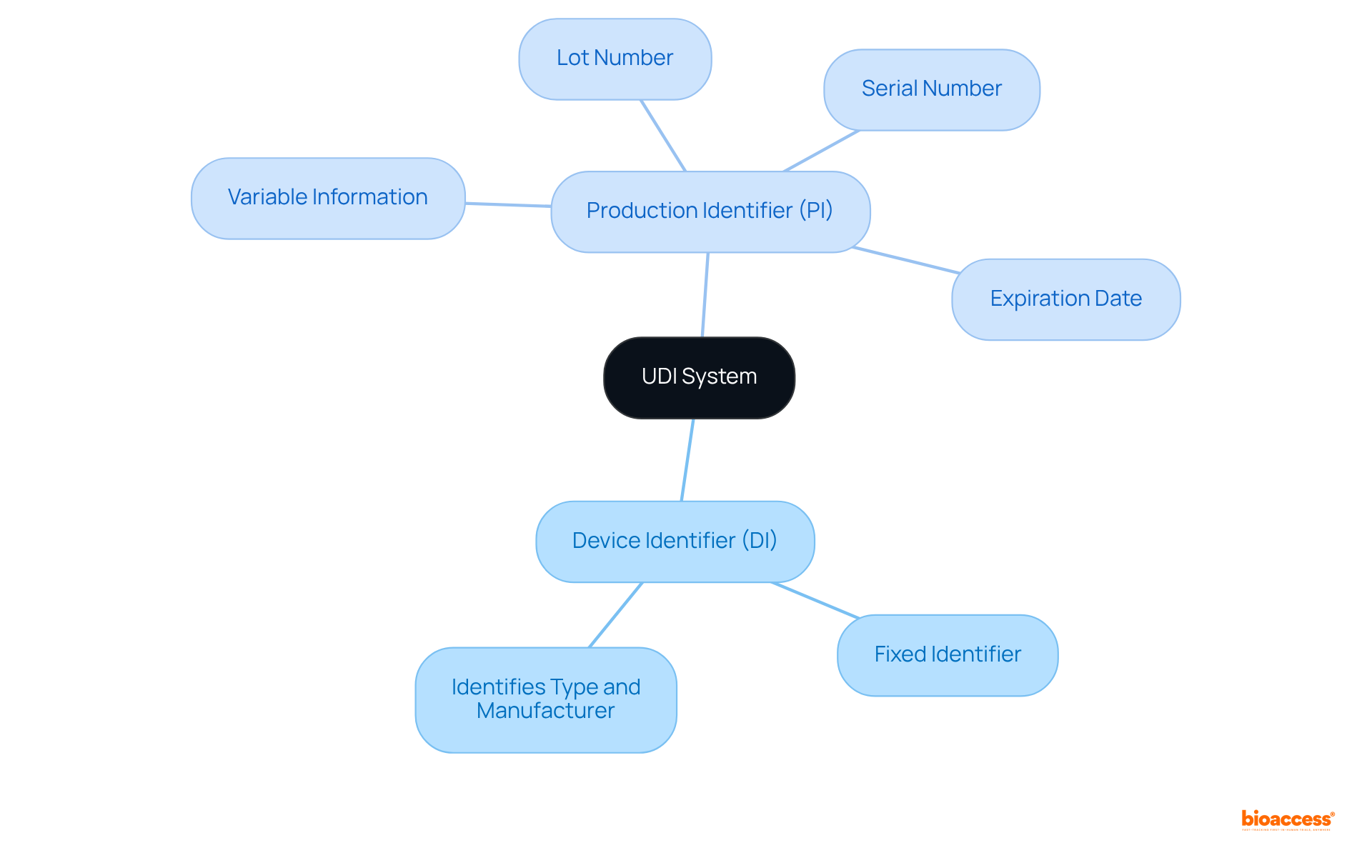
The UDI system comprises two essential components: the Device Identifier (DI) and the Production Identifier (PI). The DI serves as a fixed identifier for a specific unit, whereas the PI contains variable information, including the lot number, serial number, and expiration date.
For effective implementation, it is imperative that the UDI be displayed in both human-readable and machine-readable formats, such as barcodes or QR codes. This dual-format requirement not only streamlines inventory management but also significantly enhances patient safety by ensuring accurate identification of equipment.
Currently, a substantial portion of medical equipment utilizes both formats, highlighting what is a UDI compliance in contemporary healthcare. Experts assert that the DI is crucial for identifying the type and manufacturer of the equipment, while the PI provides vital information necessary for monitoring and managing items throughout their lifecycle.
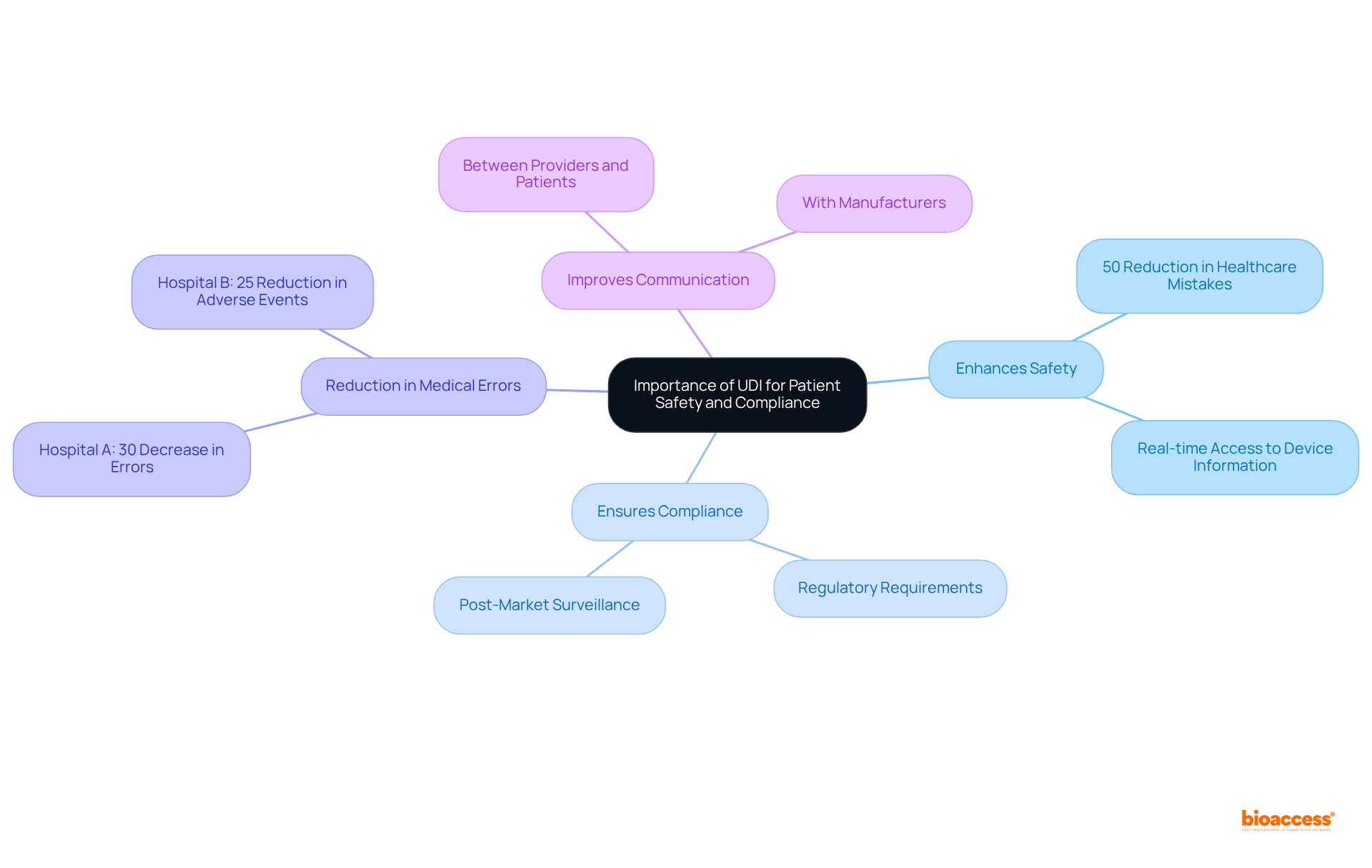
Understanding what is a udi is crucial for enhancing safety and ensuring compliance with regulatory standards through the implementation of the Unique Device Identification system. What is a udi provides precise identification of healthcare devices, allowing professionals to swiftly access vital information, such as usage history and recall status. This capability has been shown to reduce healthcare mistakes by up to 50%, according to a study published in the Journal of Patient Safety, thereby significantly improving the quality of care for patients.
For instance, hospitals that have adopted UDI, including Hospital A and Hospital B, reported a 30% decrease in medical errors and a 25% reduction in device-related adverse events. Furthermore, understanding what is a udi strengthens post-market surveillance and streamlines the reporting of adverse events, contributing to a safer healthcare environment. The UDI system not only aids in meeting regulatory requirements but also enhances communication among healthcare providers, patients, and manufacturers, ultimately leading to better healthcare outcomes.
The Unique Device Identifier (UDI) system represents a transformative approach in healthcare, ensuring that medical instruments are accurately identified and tracked throughout their lifecycle. By standardizing the identification process, UDI enhances patient safety, streamlines supply chain management, and supports regulatory compliance. As the integration of UDI continues to grow, its significance in safeguarding health outcomes and improving operational efficiencies cannot be overstated.
Key insights from the article illustrate the evolution of UDI, from its inception driven by safety concerns to its current status as an essential component of healthcare practices. The UDI system, consisting of the Device Identifier (DI) and Production Identifier (PI), plays a crucial role in reducing medical errors, improving recall effectiveness, and facilitating better communication among healthcare stakeholders. Real-world examples, such as those from Duke University Health System, further highlight the substantial benefits that UDI brings to patient care.
In light of these developments, it is imperative for healthcare professionals and organizations to fully embrace the UDI system. By prioritizing UDI implementation and fostering awareness of its benefits, the healthcare community can enhance safety measures, reduce errors, and ultimately improve patient outcomes. The ongoing commitment to UDI not only fulfills regulatory requirements but also represents a proactive step towards a safer and more efficient healthcare environment.
What is a Unique Device Identifier (UDI)?
A UDI is a standardized numeric or alphanumeric code assigned to medical instruments, designed to uniquely identify them throughout their lifecycle.
What are the two main components of a UDI?
The two main components of a UDI are the Device Identifier (DI), which specifies the particular version or model of an apparatus, and the Production Identifier (PI), which provides additional details such as the lot or serial number.
Why is the UDI system important in healthcare?
The UDI system is crucial for ensuring traceability and safety in healthcare environments, enabling better understanding and management of the equipment used in patient care.
What is the projected adoption rate of UDI in medical instruments by 2025?
By 2025, it is projected that approximately 80% of medical instruments will incorporate UDI.
How does the FDA view the implementation of UDI?
The FDA is committed to UDI implementation, recognizing its potential to improve health outcomes and operational efficiencies in healthcare.
Can you provide an example of successful UDI implementation?
An example of successful UDI implementation is at Duke University Health System, where it has led to substantial improvements in recall responses and overall patient safety.
What challenges exist regarding UDI implementation?
Challenges include the need for stronger policy support and increased awareness of UDI and its benefits among healthcare leaders.
What role does the AccessGUDID database play in the UDI system?
The AccessGUDID database provides a centralized resource for identification and tracking of medical devices, enhancing clinical practice and illustrating the importance of UDI.