


The Investigational New Drug (IND) designation is pivotal for drugs or biological products that have yet to gain FDA approval for general use. This designation is a critical milestone in clinical studies, aimed at evaluating both safety and effectiveness.
The IND submission process encompasses comprehensive details regarding the drug and its proposed trials, which the FDA meticulously reviews to uphold ethical standards and ensure participant safety. This rigorous evaluation ultimately facilitates the transition from laboratory research to market readiness, underscoring the importance of this process in the realm of clinical research.
Understanding the intricacies of drug development is crucial in an era where medical innovation is paramount. The Investigational New Drug (IND) application serves as a pivotal gateway for new therapies, allowing researchers to transition from laboratory discoveries to clinical trials with human subjects. However, the complexities of the IND process raise important questions:
Delving into these aspects reveals not only the significance of the IND in advancing healthcare but also the challenges that accompany regulatory compliance in the pharmaceutical landscape.
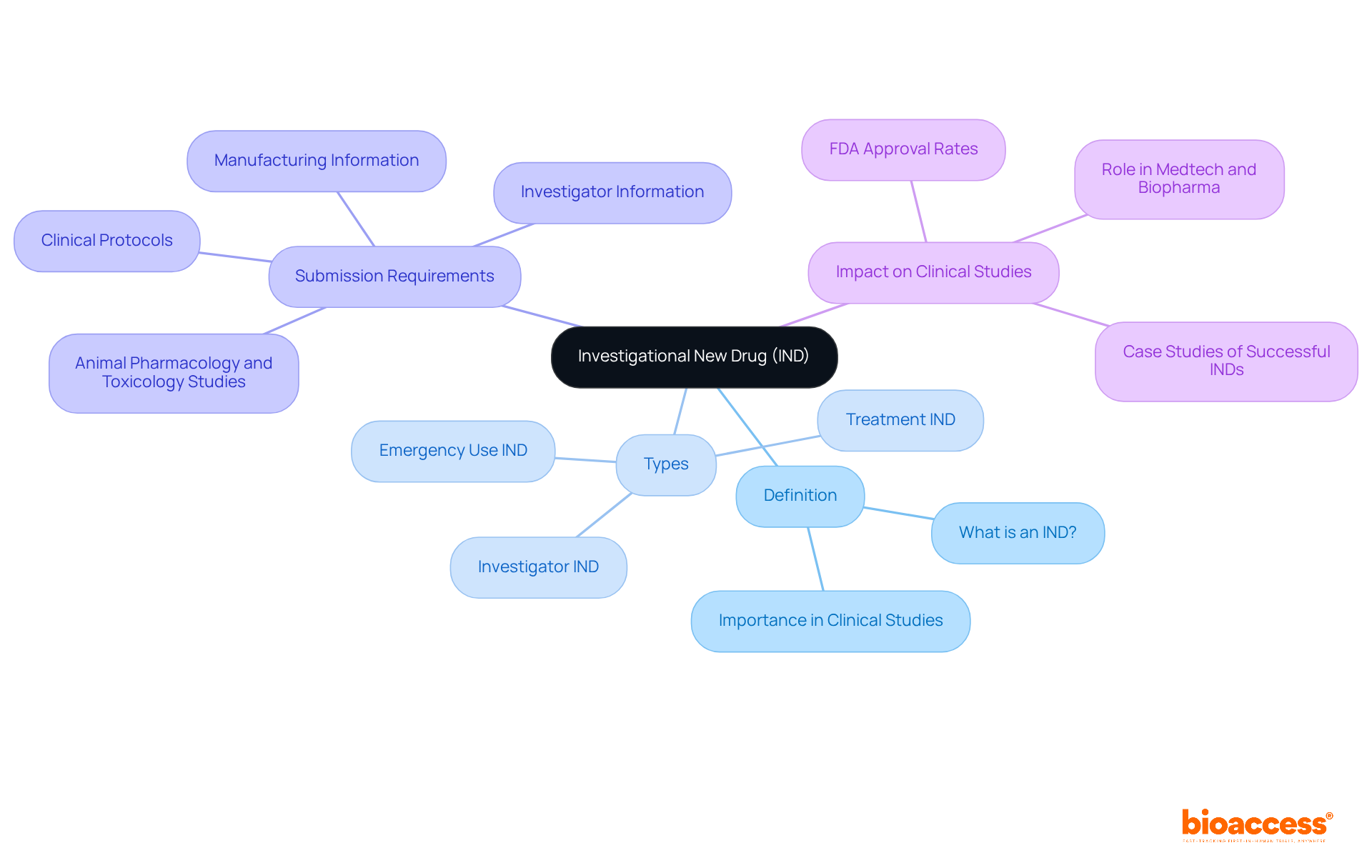
What is an IND refers to an Investigational New Drug, which is a drug or biological product that has yet to receive approval for general use by the U.S. Food and Drug Administration (FDA). This designation is pivotal in clinical studies designed to evaluate the safety and effectiveness of new therapies. The IND submission acts as a formal request to the FDA, seeking to clarify what is an IND for authorization to commence clinical trials involving human subjects. This process is essential for researchers to gather critical data regarding the drug's effects and potential side effects, ensuring that new medications are both safe and effective before their market introduction.
What is an IND refers to three distinct types: Investigator IND, Emergency Use IND, and Treatment IND. Each type fulfills a specific role within the clinical research landscape. The IND submission must encompass comprehensive details across several vital areas, including what is an IND related to Animal Pharmacology and Toxicology Studies, Manufacturing Information, Clinical Protocols, and Investigator Information. This complexity highlights the stringent standards that must be adhered to for the sake of public safety.
In recent years, the FDA has shown a commitment to streamlining the IND process, essentially addressing what is an IND, with approximately 80% of IND applications gaining approval. This statistic underscores the agency's recognition of the IND's crucial function in improving medication safety and efficacy. As noted by FDA officials, understanding what is an IND process is fundamental for safeguarding public health while promoting innovation in drug development.
Successful IND applications have played a significant role in the Medtech and Biopharma sectors, enabling the launch of transformative therapies. For instance, bioaccess® offers comprehensive clinical study management services, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting across Latin America, Eastern Europe, and Australia. This expertise empowers Medtech, Biopharma, and Radiopharma startups to navigate the IND process effectively, ultimately leading to accelerated clinical studies and significant advancements in treatment alternatives. Such approvals not only emphasize the importance of the IND process but also reflect ongoing efforts to enhance patient care through innovative medical solutions.
The Investigational New Drug (IND) request, which is essential for understanding what is an IND, stands as a cornerstone of the pharmaceutical development process, serving as the crucial gateway for new therapies to enter clinical studies. Following thorough preclinical research that confirms a medication's safety for initial human use, pharmaceutical firms must submit what is an IND to the FDA. This submission includes extensive information regarding the medication, such as its chemical structure, production methods, and proposed clinical trial protocols.
The FDA meticulously reviews the IND to ensure that the proposed studies uphold ethical standards and safeguard the rights and safety of participants. What is an IND highlights its importance, as without an approved IND, a medication cannot legally undergo human testing, making it crucial for transitioning from laboratory research to market readiness.
The timely submission and approval of IND applications can significantly influence drug development timelines, often determining the pace at which new therapies can be introduced to patients. As highlighted by industry leaders, the IND process not only facilitates the commencement of clinical studies but also plays a crucial role in ensuring that innovative therapies are developed responsibly and efficiently.
With bioaccess®'s comprehensive clinical trial management services—encompassing feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—the IND process can be streamlined. By leveraging bioaccess®'s expertise, companies can achieve 50% faster patient enrollment and realize savings of $25K per patient with FDA-ready data, effectively overcoming regulatory challenges and accelerating approval processes for startups in the medtech and biopharma sectors.
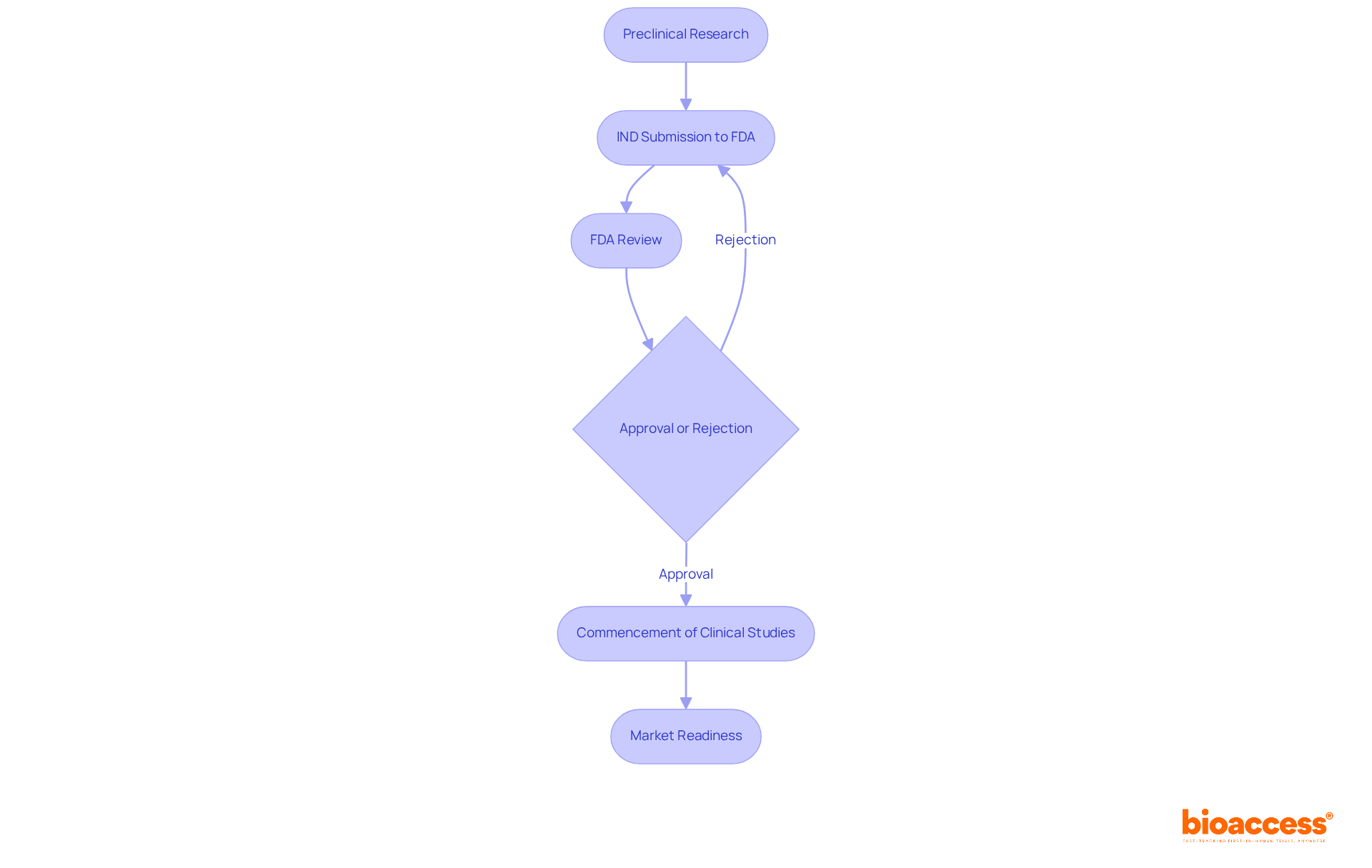
The submission process known as what is an IND emerged in the mid-20th century as a crucial response to the increasing demand for stringent pharmaceutical regulation. Initially framed by the Federal Food, Cosmetic, and Pharmaceutical Act of 1938, it was the thalidomide tragedy of the 1960s that underscored the necessity for enhanced safety measures. This catastrophic event, where the sedative resulted in severe birth abnormalities, propelled the FDA to institute the IND submission process, ensuring that new medications undergo comprehensive evaluations for safety and effectiveness prior to market introduction.
In the wake of the thalidomide incident, the FDA refined what is an IND process, introducing various application types that cater to the diverse needs of medication development. Research INDs were established for unapproved substances, facilitating controlled studies, while emergency INDs were designed to expedite access to potentially life-saving therapies in urgent circumstances. These adaptations reflect the dynamic landscape of public health and the regulatory framework established to safeguard it.
Key milestones in the evolution of IND regulations encompass the implementation of stringent preclinical testing requirements and the introduction of annual reporting obligations for sponsors. The tragic outcomes of early trials, such as the TeGenero study, further shaped regulatory modifications, leading to more cautious dosing strategies and improved monitoring protocols.
Experts in the field have noted that the evolution of the IND process has been instrumental in shaping modern medication development. As one regulatory historian remarked, 'The IND application process has transformed from a basic framework into a comprehensive system that prioritizes patient safety while facilitating innovation.' This continuous evolution highlights the FDA's dedication to adapting regulations in response to emerging challenges in drug development, ensuring that safety remains a priority as new therapies are launched into the market.
In this context, bioaccess emerges as a leader in Medtech clinical research across Latin America, offering a wide range of comprehensive clinical study management services. These services encompass feasibility studies, compliance reviews, project management, trial setup, import permits, and reporting. By ensuring meticulous adherence to regulatory requirements, bioaccess adeptly navigates what is an IND submission process, addressing the challenges posed by the evolution of drug development and enhancing the safety and efficacy of new drug innovations.
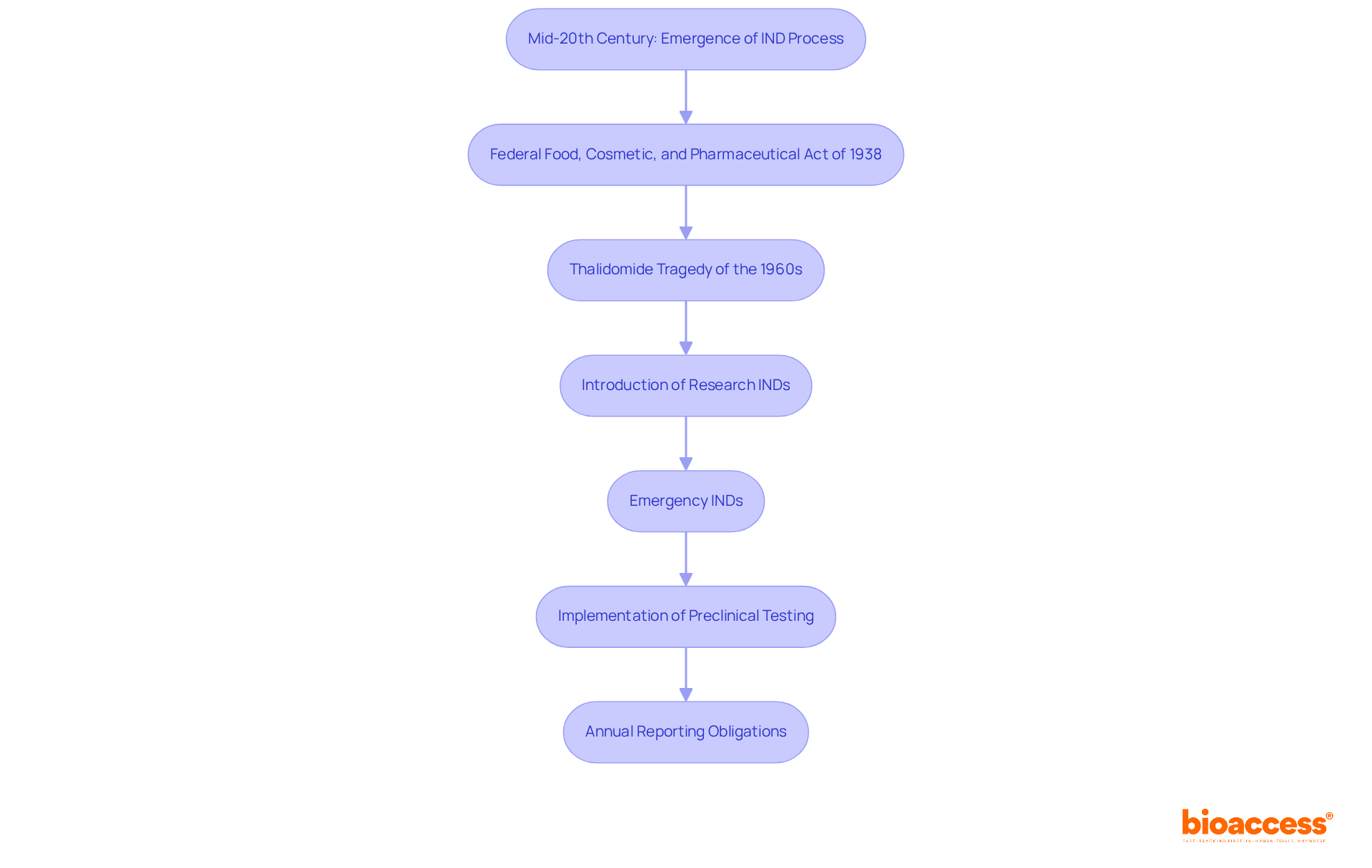
To understand what is an ind, it is important to know that an IND submission must include several key components to be considered by the FDA. These components typically consist of:
These components are crucial for the FDA to assess what is an ind, ensuring that all necessary information is provided for a thorough review of the safety and feasibility of the proposed clinical trials.
The Investigational New Drug (IND) process is a cornerstone in the development of new medications, acting as the essential gateway for innovative therapies to progress from laboratory research to clinical trials. By ensuring that drugs undergo stringent evaluation for safety and efficacy, the IND process is pivotal in safeguarding public health while promoting advancements in medical treatment.
This article has examined key elements of the IND process, including:
The FDA's dedication to streamlining this process, along with the expertise offered by organizations like bioaccess®, underscores the significance of efficient IND applications in hastening the development of transformative therapies.
Understanding the importance of the IND process is crucial for all stakeholders involved in drug development. As the pharmaceutical landscape continues to evolve, it is imperative for these individuals to stay informed and actively participate in the IND submission process to facilitate the rapid introduction of safe and effective treatments to the market. Embracing this knowledge not only amplifies the potential for innovation in healthcare but also reinforces our collective responsibility to prioritize patient safety throughout every phase of drug development.
What does IND stand for and what is it?
IND stands for Investigational New Drug, which refers to a drug or biological product that has not yet received approval for general use by the U.S. Food and Drug Administration (FDA). It is a designation essential for conducting clinical studies to evaluate the safety and effectiveness of new therapies.
What is the purpose of an IND submission?
The IND submission serves as a formal request to the FDA for authorization to begin clinical trials involving human subjects. It is crucial for researchers to collect important data on the drug's effects and potential side effects, ensuring that new medications are safe and effective before being introduced to the market.
What are the different types of IND?
There are three distinct types of IND: Investigator IND, Emergency Use IND, and Treatment IND. Each type has a specific role within the clinical research landscape.
What information must be included in an IND submission?
An IND submission must include comprehensive details regarding Animal Pharmacology and Toxicology Studies, Manufacturing Information, Clinical Protocols, and Investigator Information, reflecting the stringent standards that must be met for public safety.
How has the FDA improved the IND process in recent years?
The FDA has worked to streamline the IND process, resulting in approximately 80% of IND applications gaining approval. This indicates the agency's recognition of the importance of the IND in enhancing medication safety and efficacy.
Why is understanding the IND process important?
Understanding the IND process is fundamental for safeguarding public health while promoting innovation in drug development, as it plays a crucial role in the approval of new therapies.
How do successful IND applications impact the Medtech and Biopharma sectors?
Successful IND applications have enabled the launch of transformative therapies in the Medtech and Biopharma sectors, leading to accelerated clinical studies and significant advancements in treatment alternatives.
What services does bioaccess® provide related to the IND process?
Bioaccess® offers comprehensive clinical study management services, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting across various regions, helping startups navigate the IND process effectively.