


The principal investigator (PI) plays an indispensable role in clinical research, tasked with the design, implementation, and management of studies, all while ensuring adherence to regulatory and ethical standards. Effective PIs are required to navigate a myriad of complex challenges, including:
This underscores their vital role in fostering collaboration and maintaining data integrity, which are essential for achieving successful study outcomes. The importance of the PI's leadership cannot be overstated, as it directly influences the integrity and efficacy of clinical trials.
Understanding the role of a principal investigator (PI) is crucial for anyone involved in clinical research, as this individual serves as the cornerstone of successful study execution. PIs are responsible for everything from designing the study to ensuring compliance with ethical standards, making their leadership indispensable to the research team.
However, despite their pivotal role, many physicians transitioning into this position encounter unexpected challenges. This raises a critical question: what does it truly take to excel as a principal investigator in today's complex regulatory landscape?
What's a principal investigator is pivotal to the successful execution of clinical trials at study sites. This individual is tasked with the comprehensive design, implementation, and management of the study, ensuring adherence to all regulatory requirements and ethical standards. The PI's responsibilities encompass:
Their leadership is essential for fostering a collaborative environment among the research team, which typically includes co-investigators, research coordinators, and other key staff members.
In the realm of advancing medical device studies, the PI's role is further underscored by the extensive services offered by bioaccess, which encompass:
These services are crucial for navigating the regulatory challenges that medical device startups often face, ensuring that evaluations are conducted efficiently and effectively.
The authority and responsibility of the PI position them as a central figure in the success of research studies. For instance, Dr. Robert Springer led a Phase 3 gout trial that exceeded enrollment targets by 200%, illustrating the impact of strong leadership in achieving medical study goals. Similarly, Dr. Melita Tate's team at Grassroots Healthcare excelled in a global Phase 2 study focused on obesity, where the Tulsa site outperformed enrollment targets, highlighting the significance of a PI's role in securing successful outcomes.
Statistics emphasize the importance of PIs in medical studies; for example, public awareness of medical experiments has increased since the COVID-19 pandemic, leading to heightened involvement and interest. However, the transition to a role where one understands what's a principal investigator can be unexpectedly challenging for physicians, often necessitating more than just a background in reviewing journal articles. Expert insights indicate that effective PIs must adeptly navigate complex regulatory environments, including compliance reviews and import permits, while fostering team dynamics to ensure trial success. Ultimately, the PI's guidance and commitment are vital for advancing medical studies and achieving meaningful results.
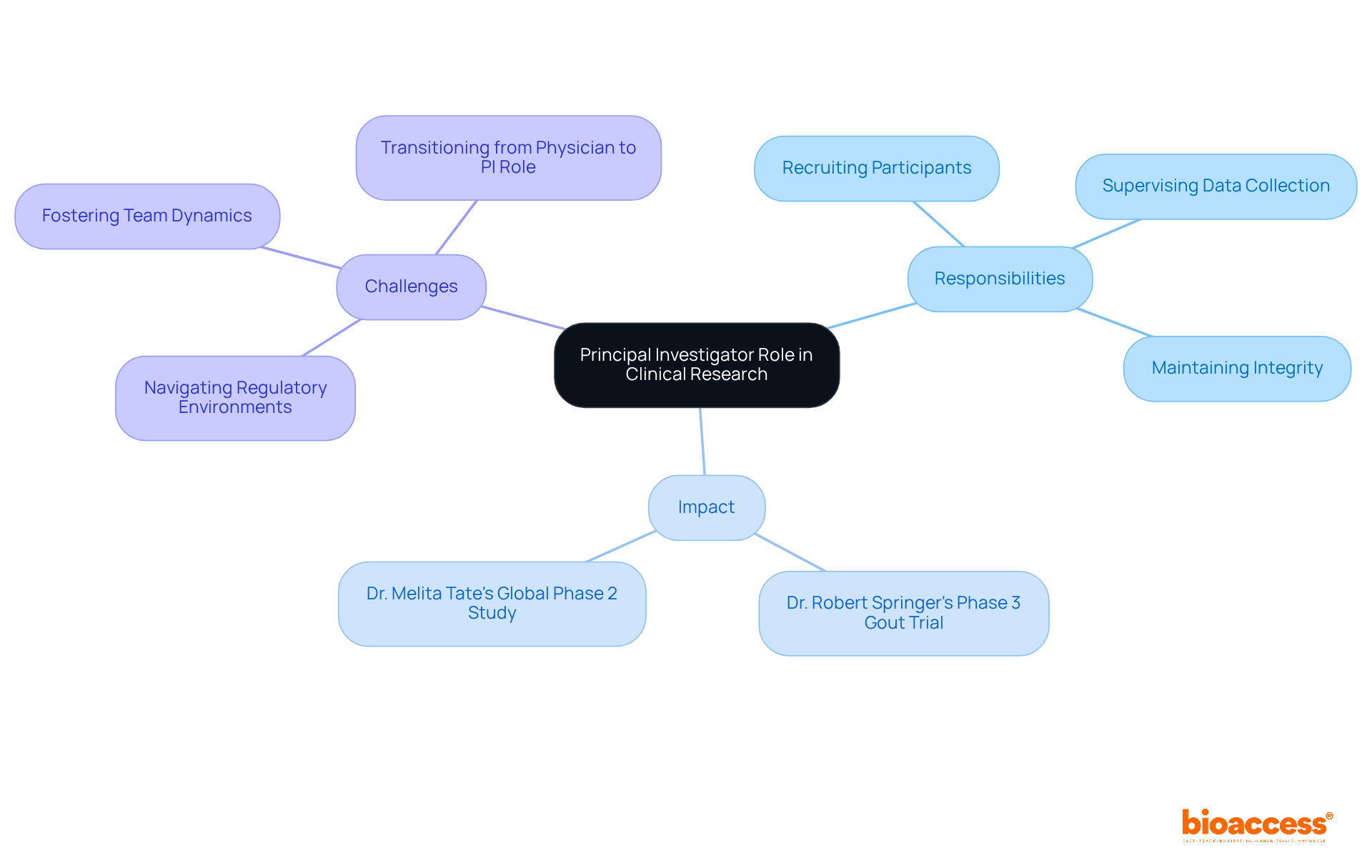
The role of what's a principal investigator (PI) has undergone significant transformation since the inception of medical research. Initially, trials were often conducted by individual researchers with minimal oversight, resulting in inconsistencies in study execution and ethical standards. However, as medical studies have become more complex and regulatory frameworks have tightened, it's increasingly apparent what's a principal investigator and the necessity for a dedicated one. The introduction of formal guidelines, such as the Declaration of Helsinki and Good Clinical Practice (GCP), has profoundly influenced the PI's responsibilities, mandating a higher level of accountability and ethical conduct.
Today, understanding what's a principal investigator includes not only possessing deep scientific knowledge but also demonstrating robust leadership and management capabilities. This shift reflects a broader trend towards enhanced transparency and accountability in investigative practices, ensuring that patient safety and data integrity are prioritized. The evolving landscape of medical studies, which includes the rise of decentralized research and the integration of technology, has rendered the PI's role more complex, necessitating flexibility and ongoing education.
Moreover, the timeline of changes in PI responsibilities illustrates a clear trajectory towards increased oversight and regulatory compliance. For instance, the implementation of stringent documentation requirements and the necessity for timely communication with Institutional Review Boards (IRBs) have become critical components of the PI's duties. As the medical investigation setting continues to evolve, understanding what's a principal investigator's role is likely to expand further, underscoring the importance of ethical leadership and operational excellence in navigating the challenges of contemporary trials.
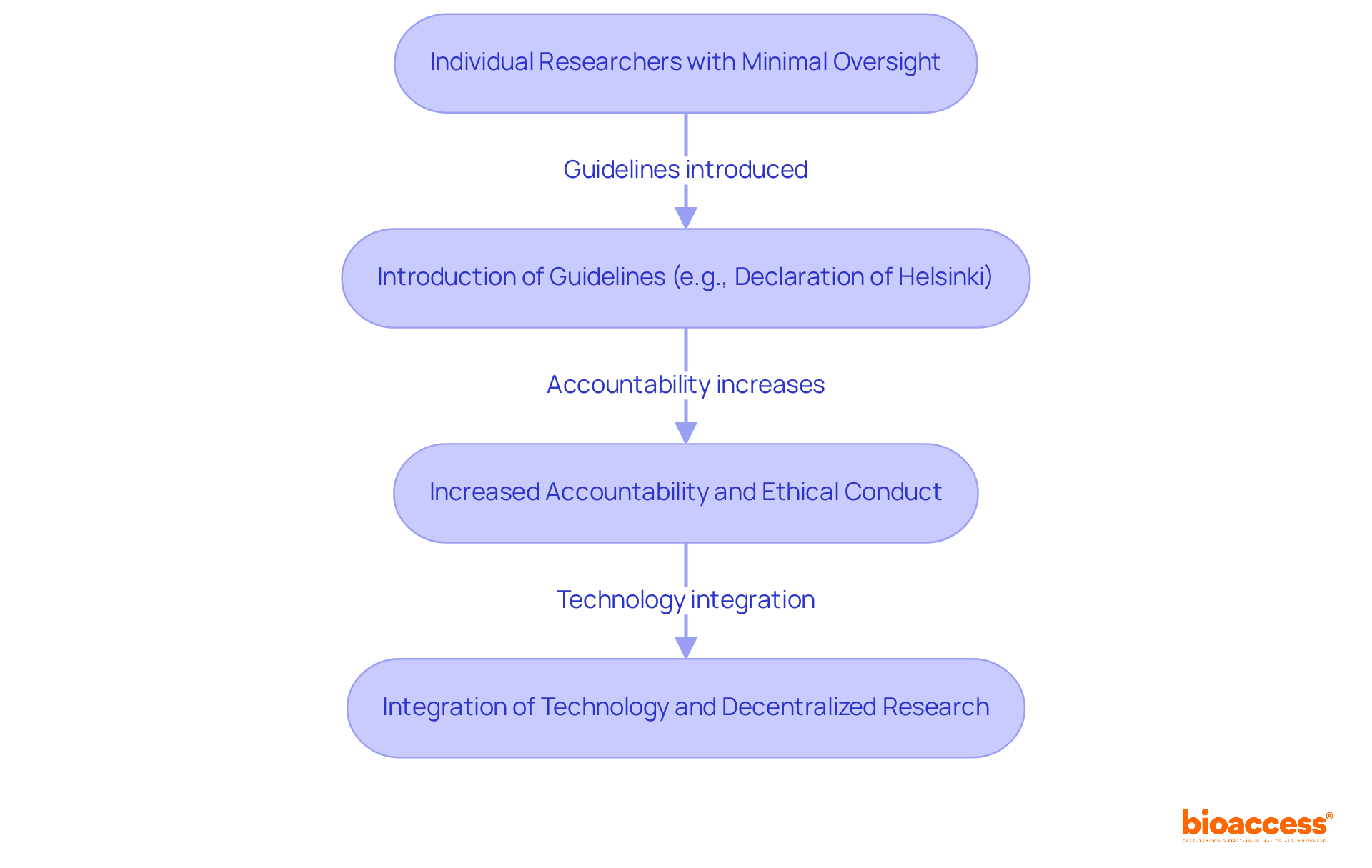
The role of what's a principal investigator (PI) is crucial in clinical studies, embodying a range of responsibilities that are vital to the success of any investigation. Among these key responsibilities are:
To understand what's a principal investigator, it's important to note that effective individuals in this role typically exhibit strong leadership traits, exceptional communication skills, meticulous attention to detail, and a profound understanding of medical study methodologies. Their ability to balance scientific precision with ethical considerations is crucial for the success of medical studies. Effective PIs are characterized by their proactive approach to problem-solving and their commitment to fostering an ethical investigative environment. This combination of skills and attributes not only elevates the quality of research but also contributes to the overall integrity of clinical trials.
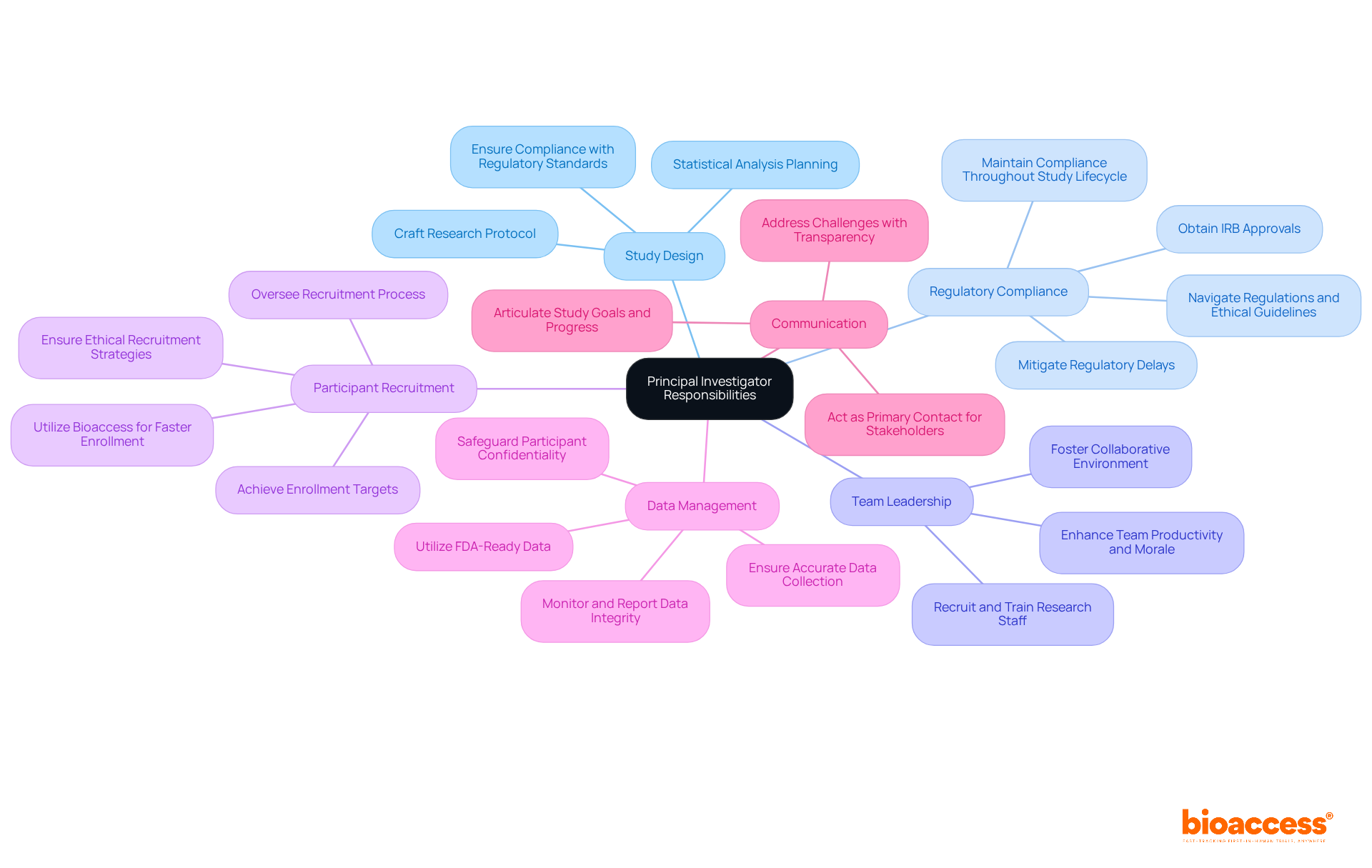
In clinical research, what's a principal investigator? They face a multitude of challenges that significantly impact their effectiveness and the success of their studies.
Regulatory Hurdles: Navigating the intricate landscape of regulatory requirements can be a daunting task for PIs. The time-consuming nature of compliance often delays study initiation, with many PIs reporting feelings of inadequacy in managing these complexities. Staying abreast of evolving regulations is crucial for ensuring that studies meet ethical standards and regulatory expectations.
Funding Constraints: Securing sufficient funding remains a critical challenge, as it directly influences the scope and feasibility of research projects. To understand what's a principal investigator, it's important to note that PIs must excel in grant writing and budget management to attract financial support, with statistics indicating that funding challenges frequently obstruct clinical studies.
Participant Recruitment: Effective recruitment and retention of study participants, particularly from specific populations, pose significant hurdles. To understand what's a principal investigator, it's important to note that they are tasked with developing innovative recruitment strategies to meet enrollment targets, often requiring tailored approaches that resonate with diverse demographics.
Team Management: For PIs, knowing what's a principal investigator involves the ability to manage multiple projects and diverse teams. Without strong leadership and interpersonal skills, conflicts and inefficiencies can arise. To understand what's a principal investigator, it's essential to recognize that they must foster a collaborative environment, balancing guidance with the independence of team members to enhance productivity.
Data Integrity: Maintaining the accuracy and reliability of data collected during trials is paramount. What's a principal investigator? PIs are accountable for executing stringent monitoring and quality assurance procedures to uphold data integrity, which is essential for the credibility of study outcomes.
These challenges underscore the necessity for effective leadership qualities and strategic planning skills in PIs, which prompts the question, what's a principal investigator? Such capabilities enable them to navigate the complexities of clinical research while prioritizing participant safety and ethical compliance.
The role of a principal investigator (PI) is vital in the landscape of clinical research, serving as the cornerstone for the successful execution of studies. This multifaceted position demands not only scientific expertise but also exceptional leadership and management skills to navigate the complexities of research regulations and ethical standards. Understanding what a principal investigator entails is crucial for appreciating their impact on the integrity and outcomes of clinical trials.
Throughout the article, key responsibilities of PIs were outlined, including:
The evolution of the role has seen an increasing emphasis on accountability and ethical conduct, driven by stringent regulatory frameworks and the need for transparency in research practices. Moreover, the significant challenges faced by PIs, such as:
highlight the demands placed on these professionals in their pursuit of advancing medical knowledge.
In light of these insights, it becomes clear that the success of clinical trials hinges on the capabilities of principal investigators. As the field of medical research continues to evolve, fostering a deeper understanding of the PI's role is essential not only for aspiring researchers but also for stakeholders invested in the integrity of clinical studies. Emphasizing the importance of effective leadership, ongoing education, and strategic planning will empower PIs to overcome the challenges they face, ultimately leading to more successful and ethically conducted research.
What is the role of a principal investigator (PI) in clinical research?
The principal investigator is responsible for the comprehensive design, implementation, and management of clinical trials, ensuring adherence to regulatory requirements and ethical standards.
What are the main responsibilities of a principal investigator?
The main responsibilities of a principal investigator include recruiting participants, supervising data collection, and maintaining the integrity of the study process.
How does a principal investigator contribute to the research team?
The principal investigator fosters a collaborative environment among the research team, which typically includes co-investigators, research coordinators, and other key staff members.
What services are offered by bioaccess to support principal investigators in medical device studies?
Bioaccess offers services such as feasibility assessments, site selection, compliance reviews, study setup, import permits, project management, and reporting, which help navigate regulatory challenges.
Can you provide examples of successful principal investigators and their contributions?
Dr. Robert Springer led a Phase 3 gout trial that exceeded enrollment targets by 200%, and Dr. Melita Tate's team excelled in a global Phase 2 study on obesity, highlighting the significant impact of strong leadership in achieving study goals.
How has public awareness of medical experiments changed since the COVID-19 pandemic?
Public awareness of medical experiments has increased since the COVID-19 pandemic, leading to heightened involvement and interest in clinical research.
What challenges do physicians face when transitioning to the role of a principal investigator?
Physicians often find the transition to a principal investigator role unexpectedly challenging, as it requires more than just a background in reviewing journal articles; they must navigate complex regulatory environments and foster team dynamics.
Why is the guidance and commitment of a principal investigator vital for research studies?
The guidance and commitment of a principal investigator are crucial for advancing medical studies and achieving meaningful results, as they play a central role in the success of research studies.