


Colombia's medical device market is on an impressive upward trajectory, fueled by a combination of demographic shifts, heightened healthcare spending, and groundbreaking technological advancements. As the sector anticipates a compound annual growth rate (CAGR) of 8% over the next five years, the region is witnessing significant developments, such as innovative clinical trials and successful product implementations. However, navigating the complexities of this burgeoning market requires a nuanced understanding of regulatory frameworks and local healthcare dynamics.
This article delves into the intricacies of conducting medical device clinical trials in Colombia, exploring the essential steps, challenges, and opportunities that lie ahead for researchers and industry stakeholders.
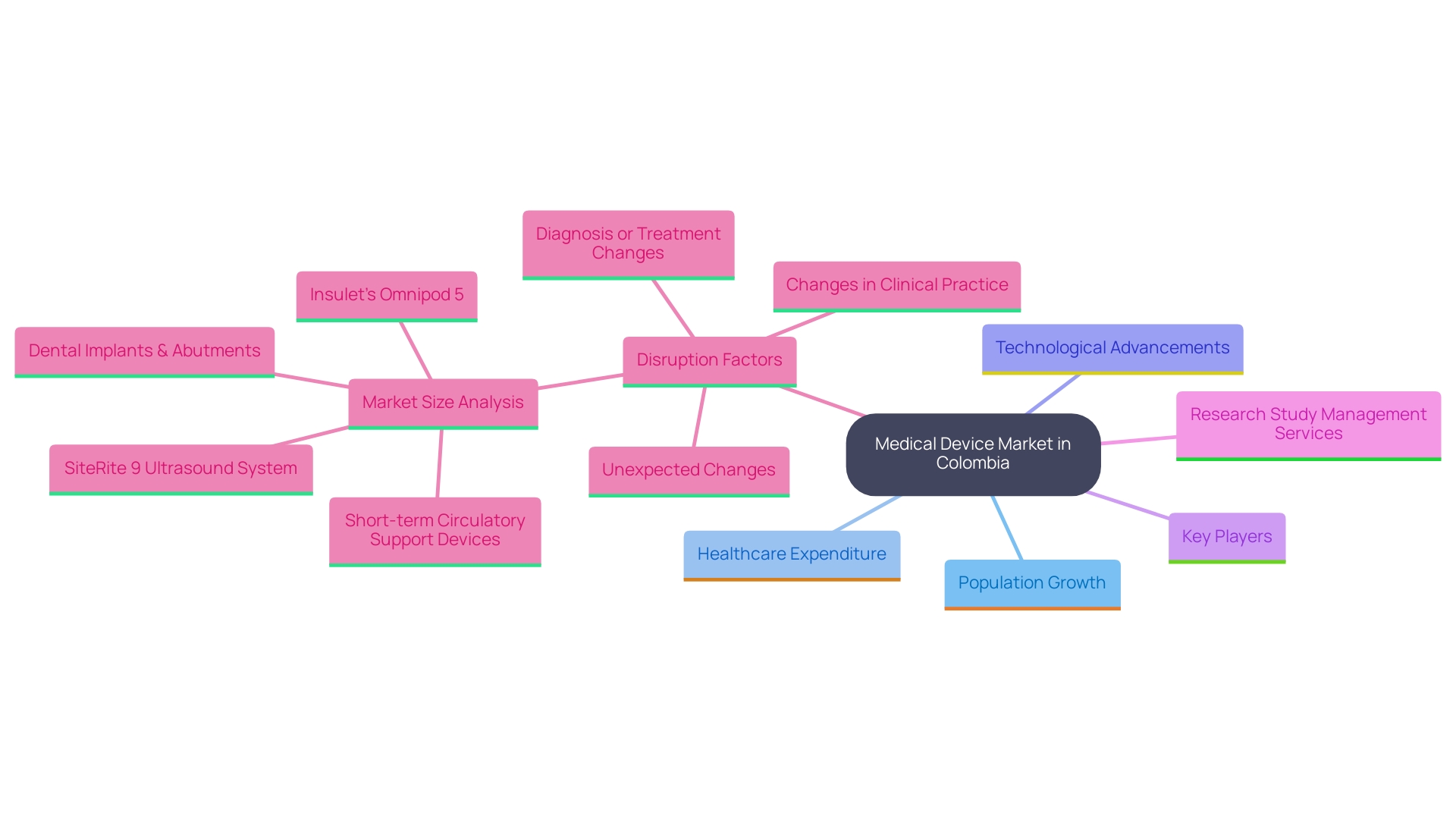
Colombia's medical device market is experiencing rapid growth, driven by a rising population, increasing healthcare expenditure, and technological advancements. For instance, the market is projected to grow at a compound annual growth rate (CAGR) of 8% over the next five years. Recent successful outcomes, such as eyeFlow, Inc.'s receipt of INVIMA approval for an 18-month pilot study on an innovative glaucoma treatment in Barranquilla, exemplify the positive trajectory of medical research in the region. Additionally, PAVmed's successful first-in-human implantations of its PortIO™ Intraosseous Infusion System in Barranquilla highlight the potential for innovative solutions in local healthcare.
Key players in this market, including both local manufacturers and international firms, must focus on compliance with INVIMA regulations to thrive. Grasping these dynamics is crucial for researchers to recognize opportunities and align their studies with market demands.
Furthermore, comprehensive research study management services—including:
are essential for navigating the complexities of research in Colombia. The cultural context and local healthcare framework also play essential roles in the success of healthcare evaluations, highlighting the necessity for customized strategies in research.
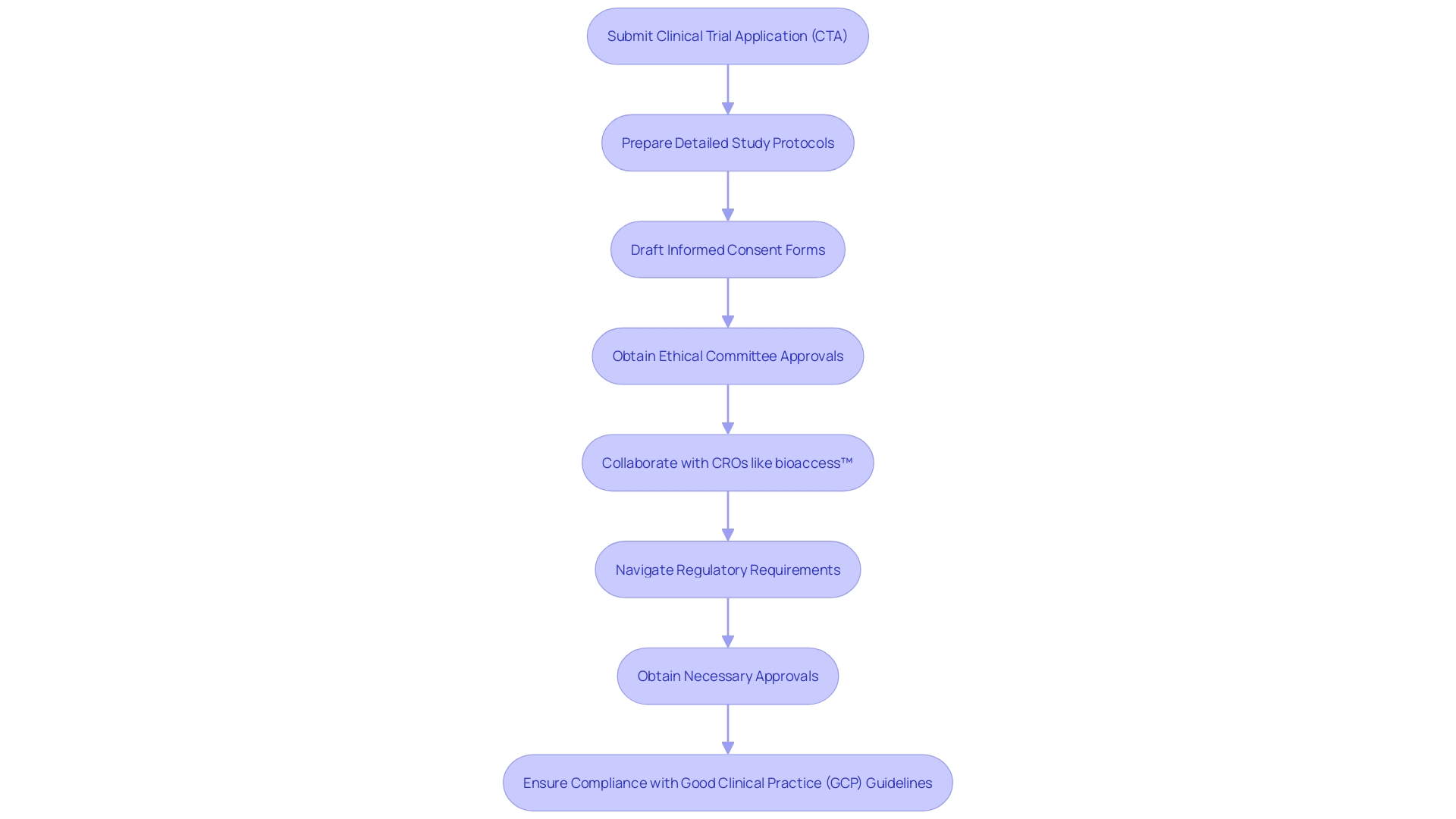
In Colombia, studies for healthcare instruments are regulated by rules set by INVIMA, the National Food and Drug Surveillance Institute, acknowledged as a Level 4 health authority by PAHO/WHO. Researchers must submit a Clinical Trial Application (CTA) that includes:
Compliance with Good Clinical Practice (GCP) guidelines is crucial to ensure ethical and scientific quality. Collaborating with bioaccess™, a vetted CRO and consulting partner for U.S. healthcare product companies, can streamline navigation of the regulatory landscape. Their comprehensive trial management services encompass:
Researchers should also be cognizant of the timelines for regulatory approvals, as these can vary significantly. Katherine Ruiz, a specialist in regulatory matters for healthcare products and in vitro diagnostics, stresses the significance of comprehending these timelines and requirements to accelerate the approval process.
Furthermore, healthcare equipment startups frequently face obstacles like:
These factors make local expertise essential for successful execution.
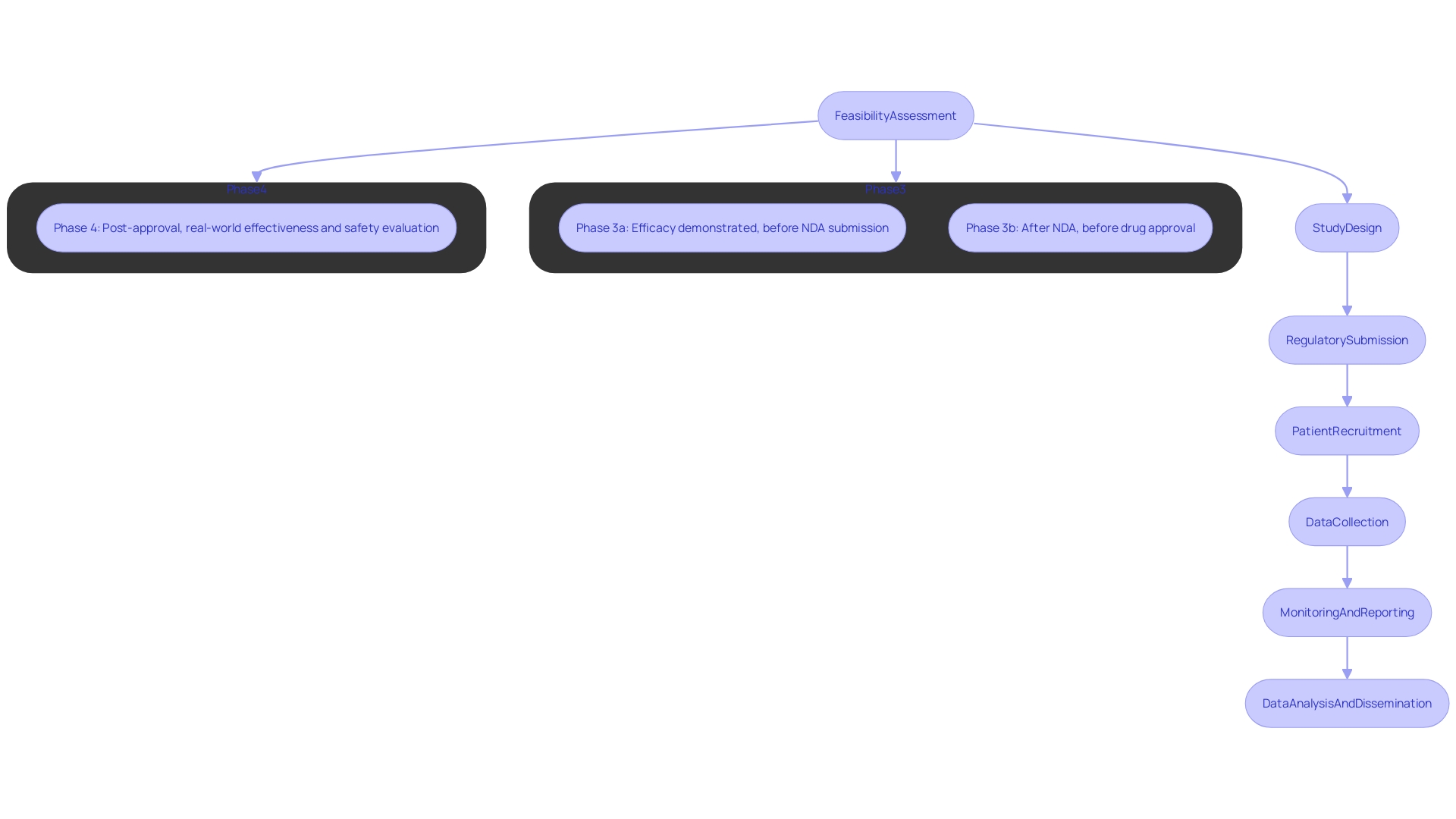
Conducting clinical trials for medical devices in Colombia involves several key steps:
Feasibility Assessment: Evaluate the target population, site selection, and study feasibility based on local conditions and resources. This is supported by bioaccess®'s expertise in feasibility and research site selection, ensuring optimal conditions for your clinical trial. For instance, in a recent project, bioaccess® identified a site that significantly improved patient recruitment timelines.
Study Design: Develop a robust study protocol that includes objectives, methodology, and statistical analysis plans tailored to the healthcare product, leveraging the specialized knowledge of our team with over 20 years in Medtech. Our previous studies have demonstrated the effectiveness of customized protocols in achieving regulatory approval.
Regulatory Submission: Prepare and submit the Clinical Trial Application (CTA) to INVIMA, Colombia's National Food and Drug Surveillance Institute, which oversees the approval process and ensures compliance with health regulations. This regulatory body is recognized as a Level 4 health authority by PAHO/WHO, ensuring rigorous standards in medical device oversight. Our team has successfully navigated the CTA process for numerous clients, expediting approval times.
Recruitment: Implement patient recruitment strategies that comply with ethical guidelines, ensuring informed consent is obtained from all participants, supported by bioaccess®'s tailored approach to recruitment. Our innovative recruitment strategies have resulted in heightened participant engagement and retention in previous studies.
Data Collection: Execute the clinical study while adhering to the study protocol, ensuring data integrity and compliance with Good Clinical Practice (GCP), monitored effectively by our project management team. Our meticulous data collection processes have consistently yielded high-quality data for analysis.
Monitoring and Reporting: Continuous observation of study progress and timely communication of adverse events to regulatory bodies as required, facilitated by our dedicated study management and reporting services. We ensure that all reporting is compliant with local regulations, which has proven essential in maintaining transparency with stakeholders.
Data Analysis and Dissemination: After completion of the study, analyze the data and prepare reports for publication, sharing findings with stakeholders and the health community, ensuring compliance with local and international standards. Our previous publications have contributed significantly to the scientific community's understanding of emerging medical technologies.
Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, plays a crucial role in navigating these processes, having extensive experience with INVIMA and a strong academic background in microbiology and quality management. Her insights have been instrumental in guiding clients through the complexities of regulatory compliance.
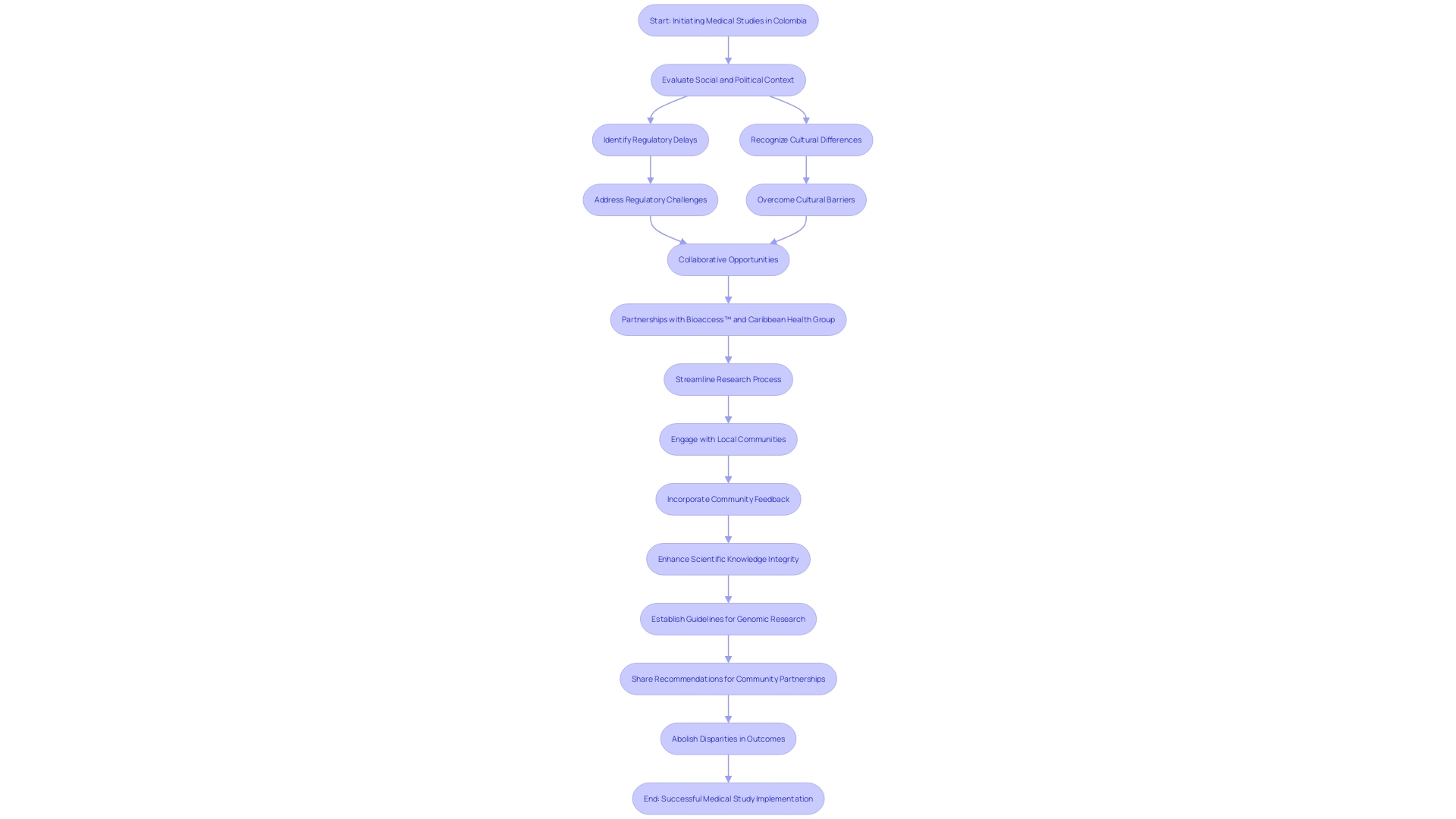
Carrying out medical studies in Colombia poses various obstacles, such as regulatory delays, cultural differences in patient recruitment, and variability in healthcare infrastructure across regions. Researchers may also encounter difficulties in ensuring compliance with local regulations. However, there are substantial opportunities, especially with the partnership between bioaccess™ and Caribbean Health Group to position Barranquilla as a leading destination for medical studies in Latin America, backed by Colombia's Minister of Health.
For example, Flow-FX's first-in-human study of its innovative Flow-Screw device for intraosseous antibiotic delivery represents a crucial step in leveraging Colombia's increasing interest in advanced medical devices. Additionally, Global Care Clinical Trials has partnered with bioaccess™ to enhance its ambulatory services in Colombia. This collaboration has achieved over a 50% reduction in recruitment time and a remarkable 95% retention rate, showcasing the effectiveness of local partnerships.
Input from leaders such as Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, emphasizes the favorable experiences with bioaccess® during research studies. He stated, "The support we received from bioaccess® was instrumental in navigating the complexities of conducting trials in Colombia." Likewise, Dr. John B. Simpson, CEO of Avinger, highlighted the satisfactory experience conducting OCT-guided atherectomy research at a location in Cali, Colombia, mentioning, "The local expertise provided by bioaccess® made our research efforts seamless and efficient."
By establishing collaborations with local participants, including healthcare organizations and practitioners, researchers can effectively address challenges and access the promising market for healthcare products.
Medical Device Contract Research Organizations (CROs) play a crucial role in clinical research by offering specialized expertise and resources that enhance clinical study efficiency. CROss in Colombia provide a comprehensive process for advancing medical device studies, including:
Their established relationships with local healthcare providers facilitate smoother interactions and enhance recruitment efforts, benefiting from a population of over 50 million with robust universal healthcare coverage. Furthermore, Colombia's cost efficiency—providing savings of over 30% compared to experiments in North America or Western Europe—and expedited regulatory processes, usually finalized within 90-120 days, make it a compelling location for first-in-human studies. Collaborating with a CRO allows researchers to focus on their core competencies while leveraging the CRO's experience in navigating the Colombian clinical research environment, ultimately leading to more successful trial outcomes.
The Colombian medical device market is poised for substantial growth, driven by demographic changes, increased healthcare spending, and technological innovations. As highlighted, the market's projected CAGR of 8% over the next five years signifies a promising landscape for clinical trials and product development. Key successes, such as the approval of innovative treatments and the establishment of strong partnerships, underscore the potential that Colombia holds for medical device research and implementation.
Navigating the regulatory framework established by INVIMA is crucial for researchers aiming to conduct clinical trials in Colombia. Understanding the complexities of the Clinical Trial Application process, adhering to Good Clinical Practice guidelines, and collaborating with experienced CROs can significantly enhance the efficiency and success of trials. The step-by-step approach outlined demonstrates that careful planning and local expertise are vital in overcoming challenges such as regulatory delays and patient recruitment hurdles.
While challenges exist, the opportunities within the Colombian market are abundant. Collaborations between local stakeholders and international companies can lead to innovative solutions and improved clinical trial experiences. The success stories from various organizations illustrate the benefits of leveraging local knowledge and networks, ultimately contributing to a more robust medical device sector.
In conclusion, stakeholders in the medical device industry must remain informed about the evolving landscape of clinical trials in Colombia. By embracing the local context, regulatory requirements, and available resources, researchers can effectively navigate this promising market. The future of medical device development in Colombia looks bright, and with strategic approaches, it can lead to groundbreaking advancements in healthcare.
What factors are driving the growth of Colombia's medical device market?
The growth is driven by a rising population, increasing healthcare expenditure, and technological advancements, with a projected compound annual growth rate (CAGR) of 8% over the next five years.
What recent developments highlight the progress in Colombia's medical research?
Recent developments include eyeFlow, Inc.'s INVIMA approval for a pilot study on a glaucoma treatment and PAVmed's successful first-in-human implantations of its PortIO™ Intraosseous Infusion System in Barranquilla.
What role does INVIMA play in healthcare studies in Colombia?
INVIMA, the National Food and Drug Surveillance Institute, regulates healthcare studies and requires researchers to submit a Clinical Trial Application (CTA) that includes detailed study protocols, informed consent forms, and ethical committee approvals.
What are the essential services for managing research studies in Colombia?
Essential services include feasibility assessments, site selection, compliance evaluations, study setup, and project management.
What challenges do healthcare equipment startups face in Colombia?
Startups face challenges such as navigating regulatory barriers, competing with established companies, obtaining involvement from research sites, and addressing financial limitations.
What are the key steps involved in conducting clinical trials for medical devices in Colombia?
The key steps include feasibility assessment, study design, regulatory submission, patient recruitment, data collection, monitoring and reporting, and data analysis and dissemination.
How can researchers streamline the regulatory process in Colombia?
Collaborating with organizations like bioaccess™, which provides trial management services, can help researchers navigate the regulatory landscape and comply with INVIMA requirements.
What are the benefits of conducting clinical trials in Colombia?
Benefits include cost efficiency—savings of over 30% compared to North America or Western Europe—and expedited regulatory processes, typically finalized within 90-120 days.
How do Medical Device Contract Research Organizations (CROs) contribute to clinical research in Colombia?
CROs provide specialized expertise and resources, enhancing clinical study efficiency through services like feasibility assessments, regulatory compliance, study setup, and detailed reporting.
What is the significance of local partnerships in conducting medical studies in Colombia?
Local partnerships enhance recruitment efforts and help researchers navigate challenges, ultimately making it easier to access the promising market for healthcare products.